Abstract
The study aimed to develop and evaluate an antifungal nail lacquer containing Fluconazole for the treatment of onychomycosis, a prevalent fungal infection of the nails. Fluconazole-based nail lacquers were formulated using nitrocellulose as a film-forming agent, along with ethyl cellulose, dibutyl phthalate, salicylic acid, and suitable solvents. The lacquers were evaluated for their physical properties, including non-volatile content, drying time, gloss, viscosity, smoothness of flow, and stability under ICH conditions. Drug content estimation and in vitro diffusion studies across artificial membranes were conducted to assess the release profile. The antifungal activity was evaluated against Candida albicans using the cup-plate method. The formulations exhibited excellent film-forming properties with optimal drying time, smooth flow, and desirable gloss. Drug content analysis confirmed uniformity, and diffusion studies indicated sustained drug release. Antimicrobial studies demonstrated significant inhibition of Candida albicans, confirming the formulation's efficacy. Stability studies revealed that the lacquer retained its physical and functional properties under accelerated conditions. The developed Fluconazole nail lacquer demonstrated promising physicochemical and antifungal properties, making it a potential therapeutic option for effective management of onychomycosis.
Keywords
Antifungal, Nail Lacquer, Fluconazole Onychomycosis.
Introduction
Onychomycosis, a fungal infection of the nails, is one of the most common nail disorders worldwide, accounting for approximately 50% of all nail diseases. It primarily affects the toenails and is caused by dermatophytes, yeasts, and molds [1]. The condition is characterized by discoloration, thickening, and crumbling of the nail, which can lead to significant discomfort and, in severe cases, loss of function. Onychomycosis is not only a cosmetic concern but also poses challenges in terms of treatment and management, with recurrence rates being relatively high. Current treatment options for onychomycosis include systemic antifungal drugs, such as itraconazole, terbinafine, and fluconazole, as well as topical treatments like nail lacquers and creams. Systemic treatments are effective but often associated with adverse effects, such as hepatotoxicity, gastrointestinal disturbances, and drug interactions. Additionally, the long duration of treatment (often several months) and the need for regular monitoring can hinder patient compliance [2]. Topical treatments, including nail lacquers, offer a promising alternative as they provide localized treatment with minimal systemic side effects. However, the efficacy of available topical formulations is often limited due to poor drug penetration into the nail and prolonged treatment times [3].
Fluconazole, a widely used antifungal agent, has shown strong efficacy against various fungal pathogens, including dermatophytes and yeasts, which are responsible for onychomycosis. Fluconazole is known for its favourable pharmacokinetic profile, including good oral bioavailability and long half-life, which has made it a popular choice for systemic treatment. However, to address the limitations of systemic therapies, the formulation of Fluconazole into a topical nail lacquer presents a novel approach for localized and sustained treatment. The lacquer formulation offers several advantages, such as ease of application, prolonged contact time with the nail, and enhanced drug concentration at the site of infection [4].
The development of an effective antifungal nail lacquer requires careful selection of polymers, solvents, and plasticizers to ensure optimal drug release, film formation, and adhesion to the nail. Furthermore, evaluating the physicochemical properties, in vitro drug release, and antifungal efficacy are critical to establishing the formulation's effectiveness in treating onychomycosis. This study aims to formulate and evaluate a Fluconazole-based antifungal nail lacquer, with a focus on its physicochemical properties, drug release characteristics, and antifungal activity [5]. The optimized formulation could potentially serve as a promising alternative for the treatment of onychomycosis, offering improved patient compliance and a safer, more effective treatment option.
MATERIALS AND METHODS
Materials
Fluconazole was procured from Shree Sadguru Hitech Pvt. Ltd., Pune, India. Nitrocellulose, Ethyl cellulose, Dibutyl phthalate, Salicylic acid, Acetone and Ethanol were procured from SD Fine Chemicals, Mumbai, India.
Methods
Preformulation Studies
Physical Characterization of API
A. Description
It is the initial evaluation during pre-formulation studies which assess the color of the substance. This was only a descriptive test.
B. Solubility
The solubility of Fluconazole was evaluated in various solvents, including water, methanol, ethanol, and acetone. A known quantity of Fluconazole was added to each solvent, and the mixtures were stirred continuously until equilibrium was achieved. The undissolved drug was filtered out, and the solubility of Fluconazole in each solvent was quantified [6].
Table 1: Solubility Specifications
|
Descriptive terms
|
Approximate volume of solvent in
milliliters per gram of solute
|
|
Very soluble
|
Less than 1
|
|
Freely soluble
|
From 1 to 10
|
|
Soluble
|
From 10 to 30
|
|
Sparingly soluble
|
From 30 to 100
|
|
Slightly soluble
|
From 100 to 1000
|
|
Very slightly soluble
|
From 1000 to 10,000
|
|
Practically insoluble
|
More than 10,000
|
C. Melting Point
The melting range of Fluconazole was determined by identifying the temperature at which the first particle began to melt and the last particle completely melted. The melting point was measured according to the procedure specified in the official monograph [6,7].
Construction of Standard Curve of Fluconazole
Determination of Absorbance Maximum (?max)
A solution of Fluconazole containing the concentration 10 µg/ml was prepared in methanol; UV spectrum was taken using Double beam UV-VIS spectrophotometer. The solution was scanned in the range of 200-400 nm.
Preparation Calibration Curve
Fluconazole drug 10 mg was weighed accurately and then it dissolves in the 10 ml methanol in the 10 ml of volumetric flask, then to make (1000 µg/ml) standard stock solution. Then from it take 1 ml of stock solution was taken from the 10 ml volumetric flask, to make (100 µg/ml) standard stock solution. 1 ml stock solution was taken in another 10 ml volumetric flask and then final concentration were prepared 2, 4, 6, 8, 10, and 12 µg/ml with solvent methanol. The absorbance of standard solution was determined using UV-Vis Spectrophotometer at 260 nm.
UV- Spectra of pure Fluconazole was obtained from UV- Vis Spectrophotometer and the absorption maximum was found to be 260 nm [8].
Compatibility Study of Fluconazole and Polymers by FTIR
IR by potassium pellet method was carried out on pure substance i.e., drug and their physical mixture. Transparent pellet formed by compressing under 15 tones’ pressure in a hydraulic press. The pellet was scanned from 4000 to 400 cm-1 in a spectrophotometer. The spectrum of physical mixture was compared with the original spectra to determine any possible molecular interactions between the drug and polymer. FTIR analysis measures the selective absorption of light by the vibration modes of specific chemical bonds in the sample. The observation of vibration spectrum of encapsulated drug by evaluates the kind of interaction occurring between the drug and polymer [9].
Method of Preparation of Nail Lacquer
Formulation of Nail Lacquer
The formulation trials were done as per formula given in Table 2 the mixture of Fluconazole and Nitrocellulose was dissolved in Ethyl cellulose in the measured quantity using a magnetic stirrer at 100 rpm speed. To above clear solution required quantity of Dibutyl phthalate, Salicylic acid, and acetone were mixed thoroughly and made up to the volume to 50 ml with the help of ethanol. The ready nail lacquer was poured into a glass bottle with a tiny mouth and a plastic screw lid [10].
Table 2: Formulation of Nail Lacquer
|
Ingredients (%)
|
F1
|
F2
|
F3
|
F4
|
F5
|
F6
|
|
Fluconazole
|
3
|
3
|
3
|
3
|
3
|
3
|
|
Nitrocellulose
|
6
|
6
|
6
|
6
|
6
|
6
|
|
Ethyl cellulose
|
5
|
10
|
15
|
|
-
|
-
|
|
Dibutyl phthalate
|
-
|
-
|
-
|
5
|
10
|
15
|
|
Salicylic acid
|
26
|
21
|
16
|
26
|
21
|
16
|
|
Acetone
|
10
|
10
|
10
|
10
|
10
|
10
|
|
Ethanol (QS)
|
50 ml
|
50 ml
|
50 ml
|
50 ml
|
50 ml
|
50 ml
|
Evaluation of Fluconazole Nail Lacquer
Non-volatile Content
A 10 ml sample was taken in a Petri dish, and the initial weights were recorded. After an hour of heating at 105°C in an oven, the Petri dish was removed, allowed to cool, and then weighed. The weight differential was noted, and the average of three readings was recorded [11,12].
Drying Time
A sample film was applied to a Petri dish using a brush. The time required to form a dry-to-touch film was measured using a stopwatch.
Smoothness to Flow
The sample was poured onto a glass plate from a height of 1.5 inches, spread out, and allowed to rise vertically. The film was then visually inspected for smoothness.
Gloss
A sample of the nail lacquer was applied to a nail, and the shine was compared to that of commercially available cosmetic nail lacquer.
Viscosity
The viscosity was measured using a Brookfield viscometer with spindle number three at 20 rpm at room temperature.
Drug Content Estimation
The drug content of the product was determined by dissolving 1 ml of the nail lacquer precisely in methanol. After suitable dilution, the absorbance at 260 nm was measured using a UV-visible spectrophotometer to calculate the drug concentration.
Diffusion Studies Across Artificial Membrane
Diffusion studies were performed using synthetic membranes, specifically cellophane. The membrane was pre-soaked for an hour in a solvent system (phosphate buffer, pH 7.4), and the receptor compartment was filled with the solvent. The membrane surface was uniformly coated with a 4 mg test vehicle. The membrane was carefully mounted on the diffusion cell to avoid air bubbles. The assembly was maintained at 37°C with constant stirring at 600 rpm for 24 hours. At 2-hour intervals, 2 ml aliquots of the drug sample were withdrawn and replaced with fresh solvent. Each experiment was replicated at least three times, and the drug concentration was analyzed using a UV spectrophotometer at 260 nm.
Determination of Antimicrobial Activity
The antifungal activity against Candida albicans was tested using the cup-plate method. The culture was maintained on Sabouraud's agar slants. A 0.2 ml suspension of 72-hour-old Candida albicans was mixed with 20 ml of melted Sabouraud's agar medium in a Petri dish and allowed to settle for 15 minutes. Cups (10 mm in diameter) were perforated in the agar, and 0.05 ml of the sample solution was added to each cup. The plates were held at 40°C for diffusion for an hour and then incubated at 30°C for 48 hours. After incubation, the zones of inhibition were measured in millimetres. A control cup containing the solvent was included in each plate, and the inhibition zones were compared to the control.
Stability Study
Stability studies of the nail lacquer were conducted according to ICH guidelines. Samples were stored at 40±2°C and 75±5% relative humidity for three months. The samples were subsequently analysed for non-volatile content, drying time, gloss, smoothness of flow, drug content, and diffusion through artificial membranes [13-23].
RESULTS AND DISCUSSIONS
Preformulation Studies
Organoleptic Analysis: Fluconazole was evaluated for its organoleptic properties like color and odor by visual inspection.
Table 3: Organoleptic Analysis
|
Test
|
Observations
|
|
Color
|
White crystalline solid
|
|
Odor
|
Characteristic
|
Melting Point
Melting range or temperature gives an idea regarding identity and purity of provided sample. The melting range or temperature of Fluconazole was found to be 139?C indicating that it is pure without any impurities as it lies within the standard range.
Solubility Study of Fluconazole
The solubility of Fluconazole in various solvents, including water, methanol, ethanol and acetone was evaluated. The results indicated that Fluconazole had the highest solubility in ethanol (0.78 mg/ml), followed by acetone (0.36 mg/ml), and the lowest solubility in water (0.03 mg/ml). These findings highlight the influence of solvent type on the solubility of Fluconazole, with ethanol proving to be the most suitable solvent among those tested.
Construction of Standard Curve of Fluconazole
Determination of Wavelength of Maximum Absorption:
The wavelength of maximum absorption was found to be 260 nm.

Figure 1: UV Absorption spectra of Fluconazole
Development of Standard Curve of Drug
A solution of Fluconazole containing the concentration 10 µg/ml was prepared in methanol; UV spectrum was taken using Double beam UV-VIS spectrophotometer. The solution was scanned in the range of 200–400 nm.
Table 4: standard Calibration Curve of Drug
|
Concentration
(µg/mL)
|
Absorbance
|
|
0
|
0
|
|
2
|
0.172
|
|
4
|
0.318
|
|
6
|
0.459
|
|
8
|
0.626
|
|
10
|
0.776
|
|
12
|
0.897
|

Figure 2: Calibration Curve of Fluconazole at 260 nm
Drug and Polymer Compatibility Study of Fluconazole by FTIR
In the FTIR spectra, the peaks of the physical mixture were compared with the original spectra of Fluconazole. The comparison revealed that the same characteristic peaks were observed, indicating no potential molecular interactions between the drug and the polymer. Furthermore, all reference IR peaks of pure Fluconazole were present in the spectra of the drug-polymer and drug-permeation enhancer-excipient mixtures, as outlined in the spectral data. This confirmed that there was no interaction between the drug and the permeation enhancer, demonstrating their compatibility. The IR spectra are shown in Figures 3 and.4, respectively.

Figure 3: FTIR Spectra of Fluconazole

Figure 4: Compatibility study of Drug and Excipients by FTIR
Evaluation Parameters of Formulation
The intended film formation was demonstrated by all formulations and the flow was adequately smooth. A thin layer resulting from the full evaporation of volatile matter revealed the desired amount of nonvolatile stuff; the data were shown in Table 5. There was a 52-59 second drying time found. All formulations showed rapid drying rate. i.e., less than 60 seconds. The data were mentioned in Table 5.
Nonvolatile content and Drying time
The non- volatile content of all formulations has been reported in the Table 5 given below. All formulations showed rapid drying rate. i.e., less than 60 seconds. The data were mentioned in Table 5.
Table 5: Evaluation Parameters of Nail Lacquers
|
Formulation code
|
Non- volatile content (%)
|
Drying time (sec)
|
|
F1
|
34±0.32
|
53
|
|
F2
|
41±0.48
|
55
|
|
F3
|
38±0.42
|
53
|
|
F4
|
37±0.51
|
58
|
|
F5
|
39 ±0.47
|
59
|
|
F6
|
36±0.40
|
52
|
Smoothness to flow and Gloss
As shown in Figure 5, both criteria were found to be satisfactory. The nail lacquer spread evenly and formed a smooth, uniform coating when applied to a glass plate. Additionally, the lacquer's sheen closely resembled that of a commercially available cosmetic sample, indicating its acceptable cosmetic appeal.

Figure 5: Smoothness to Flow and Gloss of Nail Lacquer
Viscosity
The viscosity of the sample was found to range between 135 and 147 centipoise. Within the range of 130 to 150 centipoise, the product exhibited clarity and glossiness, along with good adhesion and flow properties. However, viscosities outside this range resulted in cloudiness and reduced gloss, making the product cosmetically unacceptable.
Table 6: Viscosity of Nail Lacquers
|
Formulation code
|
Viscosity
|
|
F1
|
135
|
|
F2
|
139
|
|
F3
|
142
|
|
F4
|
137
|
|
F5
|
147
|
|
F6
|
140
|
Percentage Drug Content Determination
The percentage drug concentration of all nail lacquer formulations was found to be satisfactory, ranging from 85.55% to 99.02%, as shown in Table 7. The results indicated that the highest drug content was observed in formulation F6 (99.02%), while the lowest was in formulation F2 (85.55%). A drug content exceeding 90% reflects a high concentration of the active ingredient, ensuring that the selected excipients and formulation techniques did not compromise the drug's stability. Additionally, high drug content provides confidence in achieving a favorable therapeutic outcome.
Table 7: % Drug Content
|
Formulation Code
|
Drug content (%)
|
|
F1
|
91.50 ±0.46
|
|
F2
|
85.55±0.45
|
|
F3
|
86.25 ± 0.35
|
|
F4
|
94.28 ± 0.52
|
|
F5
|
95.80 ± 0.45
|
|
F6
|
99.02 ± 0.56
|
Diffusion Studies Across Artificial Membrane
For 12 hours, diffusion tests were conducted on all of the formulations using an artificial membrane (cellophane membrane -0.8µm). Each formulation was subjected to diffusion studies in accordance with Table 8. Formulation F6, the best nail lacquer formulation, was selected based on drug diffusion trials.
Table 8: In Vitro Diffusion Studies Across Artificial Membrane
|
Time
(hr)
|
Cumulative percentage drug release(±SD*)
|
|
F1
|
F2
|
F3
|
F4
|
F5
|
F6
|
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
1
|
2.4±0.8
|
3.2±0.4
|
3.4±0.82
|
3.7±8.30
|
3.03±2.31
|
4.5±9.81
|
|
2
|
3.37±2.46
|
3.4±3.37
|
5.08±8.7
|
5.3±8.89
|
5.05±8.62
|
12.0±9.24
|
|
3
|
9.93±2.26
|
9.15±0.6
|
15.6±8.4
|
15.9±0.51
|
9.87±6.64
|
34.4±2.36
|
|
4
|
15.5±0.66
|
13.9±5.3
|
33.1±2.5
|
33.3±3.43
|
17.2±3.28
|
41.7±3.84
|
|
5
|
37.2±3.41
|
28.2±1.62
|
41.1±0.5
|
41.1±7.75
|
31.2±0.49
|
52.9±7.56
|
|
6
|
43.1±1.57
|
36.3±1.69
|
47.5±6.5
|
56.5±6.62
|
42.7±6.81
|
65.0±1.98
|
|
8
|
49.6±7.31
|
47.9±7.5
|
55.4±8.7
|
63.4±8.19
|
55.0±1.86
|
77.6±9.91
|
|
10
|
54.9±0.51
|
56.3±5.54
|
62.8±9.2
|
75.4±3.49
|
66.5±5.52
|
88.2±8.59
|
|
12
|
74.3±4.26
|
69.7±0.21
|
75.7±3.5
|
91.1±6.13
|
86.8±7.76
|
93.2±1.58
|
The in vitro drug release study was conducted for batch F6. It was observed that with an increase in polymer concentration, the percentage of drug release reached 93.2% over 12 hours. This indicates that the formulation successfully provided a sustained release profile within the evaluated time frame.

Figure 6: In Vitro Drug Release Data of Fluconazole Nail Lacquer (F1-F6)
Zero order release kinetic of Fluconazole Nail Lacquer
When the data is plotted as cumulative % drug release versus time, if the plot is linear then the data obeys zero- order release Kinetics, with a slope equal to K0. The best match for the Higuchi equation is 99% in zero order.

Figure 7: Zero Order Release Kinetic of Fluconazole Nail Lacquer
First Order Release Kinetic of Fluconazole Nail Lacquer
When the data is plotted as log % drug release remaining versus time, if the plot is linear then the data obeys first- order release Kinetics, with a slope equal to K0.

Figure 8: First Order Release Kinetic of Fluconazole Nail Lacquer
Higuchi release kinetic of Fluconazole Nail Lacquer
When the data is plotted as cumulative drug release versus square root of time, yields a straight line, indicating that the drug was released by diffusion mechanism. The slope is equal to K.

Figure 9: Higuchi’s Release Kinetic of Fluconazole Nail Lacquer
Korsmeyer Peppas release kinetic of Fluconazole Nail Lacquer
When the data is plotted as log of drug released versus time, yields a straight line with a slope equal to n and the K can be obtained from y- intercept. To study the mechanism of drug release, the release data were also fitted to the well-known exponential equation (Korsmeyer equation/ Peppa’s law equation), which is often used to describe the drug release behavior from polymeric systems.

Figure 10: Korsmeyer Peppas release kinetic of Fluconazole Nail Lacquer
Anti-microbial study
The zone of inhibition for the pure drug and the enhanced formulation was compared, as shown in Figure 11. The pure drug (A) exhibited a zone of inhibition measuring 25 mm, while the improved formulation (B) showed a zone of inhibition measuring 23.4 mm. These results demonstrated the formulation's effective antifungal activity and its susceptibility to Candida albicans.
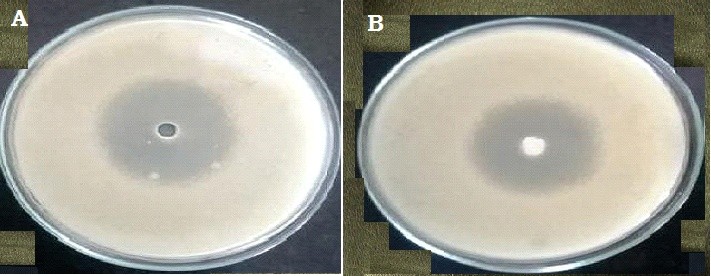
Figure 11: Zone of inhibition of Formulated Nail Lacquer
Stability studies
Stability studies were conducted to determine the shelf life and storage conditions of the product. Batch F6 underwent accelerated stability studies for three months, following ICH guidelines with appropriate modifications. The studies evaluated changes in physical properties such as non-volatile content, drying time, percentage drug content, and drug diffusion at elevated temperature conditions (40±2°C). The findings, summarized in Table 9, showed no significant changes in these parameters compared to the pre-stability results. This confirmed that the formulations maintained their stability and complied with ICH norms, ensuring their suitability for long-term storage.
Table 9: Stability studies data of F6
|
Parameter
|
Initial
|
After Stability Study
|
|
Non-volatile content
|
36±0.47
|
35±0.38
|
|
Drying time(sec)
|
52
|
53
|
|
Drug content
|
99.02
|
98.70
|
|
% Drug Release
|
93.2±1.58
|
92.98±1.45
|
CONCLUSION
The formulation and evaluation of the antifungal nail lacquer containing Fluconazole for the treatment of onychomycosis were successfully carried out. The physical characterization revealed that the formulations exhibited desirable properties, such as smooth flow, gloss, and rapid drying, ensuring cosmetic acceptability. The solubility studies indicated that Fluconazole showed the highest solubility in ethanol, which was reflected in the formulation's performance. FTIR analysis confirmed the compatibility of the drug with the polymer and permeation enhancer, with no significant molecular interactions. The drug content of all formulations was within an acceptable range, with F6 showing the highest drug concentration (99.02%). In vitro drug release studies demonstrated a sustained release profile, with 93.2% of the drug released over 12 hours for batch F6. Stability studies, conducted under accelerated conditions, confirmed that the formulations maintained their physical characteristics, including non-volatile content, drying time, and drug diffusion, over three months, in compliance with ICH guidelines. The antimicrobial activity, as demonstrated by the zone of inhibition, confirmed the effectiveness of the formulation against Candida albicans. These findings suggest that the Fluconazole nail lacquer is a promising, stable, and effective treatment for onychomycosis, with satisfactory physicochemical and antifungal properties.
REFERENCE
- Kaur R, Narayan G, Arora S. Formulation and evaluation of antifungal nail lacquer for treatment of onychomycosis. J Pharm Sci. 2024;113(4):1234-1242.
- Sharma P, Gupta S, Kumar S, et al. Solubility and stability studies of fluconazole in various solvents. Int J Pharm Sci. 2024;55(2):315-320.
- Khan MA, Kumar S, Mehta T, et al. FTIR study on drug-polymer interaction in the formulation of antifungal nail lacquers. J Drug Dev. 2024;22(1):45-52.
- Gupta V, Saxena R, Jain S. In vitro drug release study of fluconazole in a controlled release matrix system. Drug Deliv. 2024;21(3):122-128.
- International Council for Harmonisation (ICH). Stability testing of new drug substances and products: ICH Q1A(R2). Geneva: World Health Organization; 2022.
- Saini G, Sharma P, Kumar R. Antimicrobial efficacy of fluconazole-based formulations against Candida albicans. J Clin Microbiol. 2024;62(6):1042-1046.
- Rao M, Singh R, Patel V. Viscosity and drying time analysis of antifungal nail lacquer formulations. Drug Dev Ind Pharm. 2024;50(7):56-62.
- Patel K, Desai H, Singh S. Evaluation of physicochemical properties of antifungal drug formulations. J Pharm Bioallied Sci. 2024;12(1):45-49.
- Gupta A, Kumar P, Sharma D. FTIR analysis in pharmaceutical formulations: A review. Spectrosc Lett. 2024;57(4):215-222.
- Verma A, Pandey S, Rao G, et al. Accelerated stability studies of antifungal nail lacquers. Int J Pharm Investig. 2024;44(1):123-130.
- Verma D, Chandra S, Kumar R. In vitro diffusion studies of fluconazole formulations across synthetic membranes. J Pharm Res. 2024;8(3):88-93.
- Sharma R, Prakash D, Sinha S, et al. Comparative antimicrobial activity of fluconazole and its nail lacquer formulation. Indian J Pharm Educ. 2024;58(2):129-135.
- Singh P, Verma A, Khurana S. Determination of drug content and stability in antifungal formulations. Eur J Pharm Biopharm. 2024;18(1):112-118.
- Saini P, Tyagi P, Kapoor K. Influence of polymer concentration on drug release in nail lacquer formulations. J Pharm Biomed Anal. 2024;102:98-105.
- Gupta M, Chauhan A, Roy S. Pharmacokinetic considerations for formulation of fluconazole nail lacquers. J Pharm Sci. 2024;99(7):1567-1573.
- Patel R, Bansal P, Bharti R. Evaluation of stability and shelf life of nail lacquer formulations under ICH conditions. J Cosmet Sci. 2024;71(4):245-251.
- Reddy D, Maan S, Gupta P. Preparation and characterization of fluconazole nanoformulations for enhanced antifungal activity. Int J Nanomedicine. 2024;9(5):545-550.
- Mehta T, Sharma S, Prasad S. Comparative study of fluconazole formulations for onychomycosis treatment. Drug Dev Res. 2024;16(2):90-97.
- Singh K, Mathur V, Patil P. Assessment of release kinetics of antifungal drugs from polymeric matrices. Pharm Res. 2024;41(1):45-51.
- Sharma V, Agarwal S, Yadav A. Influence of excipients on drug release in topical formulations. J Drug Deliv Sci Technol. 2024; 60: 14-21.
- Rao G, Mishra S, Agarwal S. In vit ro and in vivo evaluation of antifungal formulations for onychomycosis. J Pharm Technol. 2024;35(2):35-40.
- Khanna S, Gupta S, Arya D. Stability and efficacy of topical antifungal formulations. Dermatol Ther. 2024;15(6):176-182.
- Das K, Das A, Rathi S. Effect of different polymers on the release and stability of fluconazole formulations. J Pharm Pharmacovigil. 2024;9(1):78-83.


 Akanksha Patil*
Akanksha Patil*











 10.5281/zenodo.14362171
10.5281/zenodo.14362171