Autism spectrum disorder ASD is a neurodevelopmental disorder. It is distinguished by repetitive behaviors, trouble in social interacts and difficulty in communications. Recent research has demonstrated a strong association between gut microbiota and ASD. The purpose of this review is to highlight the relationship between gut microbiome and autism spectrum disorder and how variations in gut microbiome leads to severe psychological and autistic symptoms. Methodology used in this review is Preferred Reporting Items for Systematic Review and Meta Analysis (PRISMA) guidelines. Goggle scholar and PUBMED were used for the collection of data. This review includes a total of 37 studies from the past decade. Our findings revealed that individuals with autism disorder often had variation in their gut microbiome composition and these distinct compositions are associated with behavioral symptoms and many gastrointestinal GI problems. The connection between the gut and the brain has demonstrated encouraging capability. These connections help scientists to come up with new interventions and potential treatments. By using microbiota transfer therapy MTT, probiotics, manipulating the microbiome of the gut and giving patients excusive diets there is a possibility that we can have a positive impact in ASD patients and overcome all the social and GI problems. In conclusion, more research is needed to future study the association between gut and brain and all the underlying mechanisms that link the variations in gut microbiome to the development of ASD. Further studies can open new opportunities for far more better treatments to improve the life of people with autism disorder.
GI problems, MTT, (PRISMA), gut microbiome Autism spectrum disorder
Autism spectrum disorder is a neurodevelopmental disorder and is characterized by stereotypic or repetitive behavior, deficits in social interactions and communication. Along with behavioral abnormalities ASD patients shows metabolic impairments and seizures, anxiety and sleep deficiency (Qinrui Li, Han, Dy, & Hagerman, 2017) Recent data suggested that 1 out of every 59 children are diagnosed with ASD, with the prevalence rate of 1% globally and little variations regionally in developing countries (Ho et al., 2020). Etiology of ASD is still unclear, but it is believed that both environmental and genetic factors contribute to the disorder. The risk factors includes short nucleotide polymorphism and de novo mutations accounts for 50% of the disorder (Vuong & Hsiao, 2017). Gastrointestinal abnormalities including constipation, diarrhea, gaseousness, flatulence and abdominal pain are common symptoms associated with ASD (Xu, Xu, Li, & Li, 2019). ASD patients has 67% more neurons in cortex, 17% increased brain weight and abnormal cortical pattern (Vuong & Hsiao, 2017). More than 100 genes are implicated to have important role in ASD. Asperger's syndrome and pervasive developmental disorder are specified under ASD (De Angelis, Francavilla, Piccolo, De Giacomo, & Gobbetti, 2015).Human gastrointestinal track consists of 1 kg of micro-organisms known as gut microbiota (Q Li & Zhou, 2016) There are 9.9 million gut microbial genes (Qinrui Li et al., 2017). Gut microbiota plays a crucial role in regulation of host metabolism, digestion and synthesis of vitamins like vitamin B, co-factors, riboflavin, folates and thiamine (Vuong & Hsiao, 2017). Diet is the main driver for maintaining gut microbiota balance. Main microbiota phylum in humans are Bacteroidetes, Firmicutes phyla, Proteobacteria, Actinobacteria, Fusobacteria, and Verruco microbia phyla occurring relatively rarely(Q Li & Zhou, 2016). The imbalance of microbiota is known as dysbiosis, associated with mucosal barrier disruption leads to the production of inflammatory cytokines which disturbs many neural, endocrine and immunological pathways (Fattorusso, Di Genova, Dell’Isola, Mencaroni, & Esposito, 2019). GI microbiota has physiological bidirectional interaction with brain and gut which is known as microbial gut-brain-axis (Bundgaard-Nielsen et al., 2020). The enteric nervous system is the communication conduct between gut microbiota and central nervous system (CNS)c. The gut-CNS signaling is involved with ASDs (Fattorusso et al., 2019)The brain can also influence gut microbiota through regulating intestinal permeability, motility and secretions (Q. Zhang et al., 2021). Gut microbiota effect the level of certain neurotrophins and monoamine neurotransmitter under extreme conditions and impact the normal development of brain and plasticity (De Angelis et al., 2015). ASD patients has increased concentration of pathogenic bacteria like clostridium and reduces amount of beneficial bacteria like Bifidobacterium (Cao et al., 2021). The shift in microbiota activity increases certain interleukins that increases the activation of Th1 and Th2 pathways in ASD patients (Carissimi et al., 2019).
METHODOLOGY:
We systematically reviewed the experimental and epidemiological studies of last 10 years (2013-2023 to study the gut microbiota in autism spectrum disorder and potential treatment strategies on various databases like Google Scholar and PubMed. The key words used are “autism spectrum disorder/ ASD”, “Gut microbiota”, “Gut microbiota transplantation”, “Microbiota transfer therapy” and “ASD potential therapies” were used to retrieve relevant studies. After careful screening 1500 duplicates were excluded. All the articles before timeline were neglected. 90 articles were excluded due to insufficient details, different language, non-original research and lack of proper evidence. All the articles with positive and negative findings were included. Finally, 37 articles were reviewed following the 2020 Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA) statement.
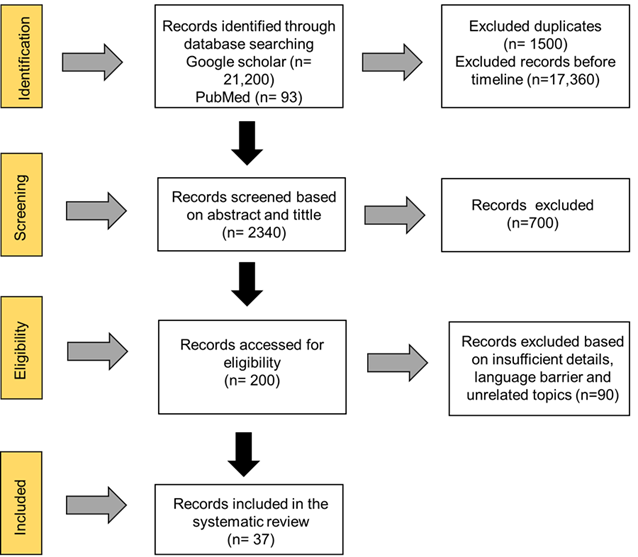
Fig 1 : Meta-Analysis Protocols (PRISMA)
- Autism spectrum disorder and Microbiota Transfer Treatment
Out of 37 studies, 3 studies were about ASD and microbiota transfer treatments. One experimental study was conducted on mouse to study the effectiveness of gut microbiota transplantation. In this study we compared the GMT original microbiota and in vitro cultured microbiota. The study reveals that both treatments significantly improved anxiety level and repetitive behaviors in ASD mouse and normalized the level of certain chemokines. Thus, the study showed that in-vitro cultured microbiota as effective as the original ones in treating ASD. 2 epidemiological studies were conducted to explore the metabolite changes in ASD children after Microbiota Transfer Therapy (MTT) that involves bowel cleansing, antibiotics and transplant of fecal microbiota (Chen et al., 2020). One study revealed that ASD patient has different metabolic profile than typically developing children. MTT leads to the significant plasma metabolites changes making them less different from controls. There wasn’t any significant difference in fecal metabolite, but p-cresol sulfate levels decreased after MTT. These findings provide evidence for MTT as a potential autism treatment and highlight the role of metabolites in the gut-brain connection (D.-W. Kang et al., 2020). In second study Author reported that a treatment called Microbiota Transfer Therapy (MTT) revealed the reduction in the gastrointestinal symptoms were seen behavioral problems in ASD patients also improved. Indicating that treatment with MTT not only improves gut symptoms but also significantly improves behavioral symptoms in ASD patients (D.-W. Kang et al., 2017).
- Probiotic, prebiotic, and symbiotic treatments on the gut microbiota in autism spectrum disorder
6 studies were to investigate the effectiveness of Probiotic, prebiotic, and symbiotic treatments on ASD patients. Out of these 6, 3 were epidemiological studies.One epidemiological showed that the combination of L. reuteri, B. longum prebiotics showed high resistance to digestive system. Increase in probiotics Lactobacillus, prebiotics increased Bifidobacterium while reducing Lachnoclostridium increased the level of beneficial SCFAs and reduced ammonium level specially with prebiotic and symbiotic treatment. This suggests that microbiota-based strategies may hold promise in managing ASD (Duque et al., 2021) (Niu et al., 2019). Another epidemiological study revealed that ASD group has lower concentration of Bacteroidetes, Bacteroides, Bifidobacterium, Ruminococcus, Roseburia, and Blautia than neurotypical group. A 4-week combination of applied behavior analysis (ABA) leads to the improvement in Autism Treatment Evaluation Checklist (ATEC) and GI scores compared to the control group with ABA training alone. While third epidemiological study Results reported that by taking probiotics for ASD patients’ improvement in anti-social behavior were observed and reduction in abdominal pain and bowel problems were observed by taking exclusive diet. These findings suggest that combining these two approaches can benefit ASD patients in both GI problems and psychological behavior (Grimaldi et al., 2018). In one experimental study agent-based model was created to study the association between gut microbiota and ASD. The study focuses on the correlation between inflammatory microbes (clostridia and desulfovibrio) and an anti-inflammatory microbe (bifidobacteria) in the gut. Findings suggest treatment with probiotics could enhance bifidobacterial that is a beneficial bacterium in the gut, and slowing the growth of a high-risk factor microbe clostridia by lysozymes reduce the risk of ASD (Weston, Fogal, Cook, & Dhurjati, 2015). While 2 experimental studies were conducted on rodents to find the association between probiotic treatments and ASD. One study was conducted on Shank3 knockout ASD mice. Discoveries showed dysregulation of Lactobacillus Reuteri. Reduction in Lactobacillus Reuteri correlates with gamma-aminobutyric acid GABA receptor expression. Unsocial behavior and repetitive behaviors were decreased in Shank3 knockout (KO) mice when treated with L. Reuteri. This finding suggests the potential role of probiotics as a beneficial tool (Tabouy et al., 2018). While the other study aimed to explore the connection between the increased use of antibiotics, gut microbiome disruption, and the rising prevalence of autism spectrum disorder (ASD) in rat’s models. They found that adult rats subjected to antibiotic treatment developed ASD-like social behavioral symptoms. The study also introduced a probiotic mixture (PM), showing that it could mitigate these behavioral symptoms induced by antibiotics and the ASD model. These results suggest that the PM has the potential to alleviate social behavioral disturbances associated with ASD, offering promise as a therapeutic intervention (Mintál et al., 2022).
- Advance sequencing techniques and autism spectrum disorder
Out of total studies, 5 were computation studies in which advanced sequencing techniques were used to explore the association between gut microbiota and ASD. A pilot study used the advanced sequencing techniques to compare ASD patients with their first-degree relatives. There was significant difference in microbial communities of ASD and neurotypical individuals. Also the development of "gut microbiome markers" is particularly promising for monitoring and improving gut health in these individuals (Kong et al., 2019). Another comparison two-staged study did fecal sample analysis by shotgun metagenomic sequencing and liquid chromatography-mass spectrometry revealed altered glutamate metabolism in the ASD group, characterized by decreased 2-keto-glutaramic acid and changes in gut microbiota associated with glutamate metabolism. Moreover, Eggerthella lenta and Clostridium botulinum was increased and Bacteroides vulgatus was in lower concentrations. Hence, gut 2-keto-glutaramic acid could serve as a potential biomarker for ASD and highlights the significant alterations in the gut microenvironment in children with ASD (M. Wang et al., 2019). One study was conducted to evaluate the impact of different primers on the microbiota analysis in ASD patients. This study revealed that different bacterial phylum’s were detected by different primers. One primer set (27f/1492r) showed significantly higher abundance of Bacteroides and a lower Firmicutes/Bacteroidetes (F/B) ratio compared to the other primer sets (515f/806r and 27f*/1495f). Another primer 27f/1492r detects higher abundance of Proteobacteria (Palkova et al., 2021). While the next study was conducted to explore the association between single nucleotide variations (SNVs) and gut microbiota in etiology of ASD. Whole genome sequencing was applied to 26 ASD and 26 controls to identify SNVs in ASD. Systematic bioinformatics analysis revealed that SNVs in innate immune response, protein glycosylation process, and retrograde axonal transport are higher in ASD patients. SNVs are involved in microbiota functioning and significantly related to ASD homeostasis (Z. Liu et al., 2021). One is experimental study in which computational model was used to integrate brain and gut metabolism to reveal the association between ASD and intestinal health. This study reveals that abnormalities in microbiota composition leads to the release of harmful toxins that can damage gut and impact functioning through gut-brain axis. they found that high fiber diet and addition of probiotic bacteria Lactobacillus acidophilus, Bifidobacterium longum longum, Akkermansia muciniphila, and Prevotella ruminicola to the diet restores gut microbiota balance and lower the oxidative stress and can be helpful in treating ASD. (Mohammad, Palukuri, Shivakumar, Rengaswamy, & Sahoo, 2022)
- Short-Chain Fatty Acids (SCFAs), Gastro-intestinal issues (GI) and autism spectrum disorder
4 studies were conducted to find the association between ASD, gastrointestinal symptoms (GI) and gut microbiota. There were 2 epidemiological studies. An epidemiological study was conducted on 90 children, 45 had ASD to investigate the relationship between GI, SCFAs and ASD. The study revealed that ASD patients are more prevalent to GI problems, but these problems had minor impact on core symptoms of ASD. Changing the level of SCFAs is associated with GI problems. Thus metabolic changes had impact on GI symptoms in ASD patients (Deng et al., 2022). While other epidemiological study was conducted on Korean children revealed that there was significant difference between both groups. ASD group showed abundance of Bacteroidetes phylum and Actinobacteria phylum and Bifidobacterium genus was in higher concentration while lower levels of the Bacteroides genus. Additionally SCFAs was also in elevated concentration in ASD patients (Ha et al., 2021). One was a rodent study. in which short chain fatty acids (SCFA) were examined to assess their role in ASD. Rodents when were treated with SCFA like butyric acid (Strati et al.) and propanoic acid (PPA) showed changes in their behavior, gene expressions and it also affected their brain functions. Moreover, PPA was observed to be involved in the triggering of genes that are responsible for the production of catecholamines and catecholamines are related to mood controls. These discoveries demonstrate that SCFA contributes to ASD through epigenetic changes in cell functions (Nankova, Agarwal, MacFabe, & La Gamma, 2014). An experimental study was conducted find the association between ASD, gastrointestinal symptoms (GI) and gut microbiota. By sequencing the bacterial 16s rRNA gene, assessing GI symptoms and detecting fecal short-chain fatty acids (SCFAs) and by analyzing they found the difference in levels of SCFAs and gut microbiota in ASD patients. Specifically, the level of acetic acid and butyrate was low but valeric acid concentration was high in ASD patients. Mostly ASD patients were constipated. Thus, modulating the gut microbiota, particularly the butyrate-producing bacteria could be a potential treatment approach for ASD (S. Liu et al., 2019).
- Variation of gut microbiome between ASD patients and controls
Out of total 16 studies were conducted on patients with ASD and controls to investigate the association between gut and brain and how much variation is present between both groups and what are the potential treatments for ASD. An epidemiological study was conducted to investigate the association between ASD and Aureobasidium pullulans (AFO-202 strain) beta glucan, known as Nichi Glucan. Total 18 children were recruited and divided into 2 groups, one receiving conventional treatment with niche glucan and other group only receiving conventional treatment. The Nichi Glucan group showed improved health by significant decrease in Enterobacteriaceae bacteria family and beneficial bacteria such as Faecalibacterium prausnitzii and Prevotella copri increased in this group. This suggests that AFO-202 beta glucan could help balance the gut microbiome in children with ASD and may also play a preventive role in neurodegenerative diseases (Raghavan et al., 2023). Following this another case control study was conducted to compare the gut microbiota composition of ASD boys with neuro-typical controls. This study reveals that certain bacterial strains like Eisenbergiella, Klebsiella, Faecalibacterium, and Blautia were significantly higher in ASD patients while Escherichia, Shigella, Veillonella, Akker mansia, Provindencia, Dialister, Bifidobacterium, Streptococcus, Ruminococcaceae UCG_002, Megasphaera, Eubac terium_coprostanol, Citrobacter, Ruminiclostridium_5, and Ruminiclostridium_6 was in lower concentration compared with neuro-typical controls. These markers can be used for early disease diagnosis (Ye et al., 2021). A pilot study backing up the above studies was conducted on 136 Chinese children, with 78 having ASD and 58 healthy controls to investigate the association between trace elements, gut microbiota and ASD. The hair sample analysis of ASD patients showed significantly higher concentration of lead, arsenic, copper, zinc, mercury, calcium, and magnesium as compared to the controls. The 16s rRAN analysis revealed increased concentration of some bacterial strains. Further analyses indicated significant associations between arsenic and mercury levels and specific gut bacteria, such as Parabacteroides and Oscillopsia (Zhai et al., 2019). Moreover, an epidemiological prospective study was conducted to investigate the development of metabolome and gut microbiota in infants with and without ASD family history for first 36 months of their life. This study reveals that infants with elevated risk of ASD (EL) has lower level of Bifidobacterium and higher levels of Clostridium and Klebsiella species compared to low-likelihood infants (Qinrui Li et al.). Additionally, LL infants excrete higher concentration of ?-aminobutyric acid (GABA) at 5 months while there wasn’t any age dependent pattern observed in EL patients. Integrated microbiome-metabolome analysis revealed a positive correlation between GABA and Bifidobacterium species and negative associations with Clostridium species (Zuffa et al., 2023). Other studies showing the variation between both groups, research reported in a cohort study that when researchers study the gut microbiota of individuals with autism, they found different bacterial patterns in autism individuals than normal individuals. A decrease in the relative abundance of Alistipes, Bilophila, Dialister, Veillonella and Parabacteroides whereas increase of Dorea, and Lactobacillus, Collinsella and Corynebacterium at genus level was observed. Similarly, Candida a fugus was found in more abundance in autism patients than in normal individuals. These results indicate that gut microbiota plays a vital role in gastrointestinal problems in individuals with autism (Strati et al., 2017). Correspondingly, Author reported in his study that when 20 autism children were compared with 20 typically developing children results showed that the gut microbiota of autistic individuals were less diverse such as Prevotella, Coprococcus, and unclassified Veillonellaceae bacteria involve in carbohydrates than typically developing children and this reduced diversity was linked with autism symptoms instead of gastrointestinal problems. All these differences in the composition of gut bacteria are related to autism symptoms (D.-W. Kang et al., 2013). Another study to back up the above findings that ASD patients show different composition of microbiota than the normal individuals reported that when gut microbiota in infants with ASD and normal infants were observed, high level of Faecali and low levels of Blautia in feces were found. Furthermore, changes in the microbiome of gut alter the expression of gene related to the immune signaling pathways in autism individuals particularly interferon-related pathways were also observed. Suggesting that changes in gut microbiota can contribute to poor function of immune system that further contributes to ASD development (Inoue et al., 2016). Likewise, Author reported in a study that when they compare the gut of children with autism with neurotypical children significant changes were found. Decrease levels of Actinobacteria and increase level of Bacteriodetes, Proteobecteria and Faecalibacterium prausnitzii were found in autistic patients suggesting an imbalance in the microbiome of the gut (Coretti et al., 2018).Supporting the above findings another Study conducted in 35 Chinese ASD children and 6 typically developing (TD) children to study their gut microbiota. They found that at genus and phylum level the ratio of certain bacteria was different in ASD than normal individuals and the level of butyrate and lactate were also low in ASD patients. These findings suggest a link between gut microbiota and pathogenesis of ASD (M. Zhang, Ma, Zhang, He, & Wang, 2018). Moreover, a study conducted on 164 children with autism spectrum disorders (ASD) also finds an association between gut microbiome and autism as many of the children were suffering from vomiting, constipation, diarrhea and bloating (V. Kang, Wagner, & Ming, 2014).Top of Form In addition, a research was conducted to assess the prevalence of Smal Intestinal Bacterial Overgrowth (SIBO) in ASD children and controls. Findings showed that children with both ASD and high SIBO showed more severe autism symptoms indicating SIBO correlation and significant role in development of autism symptoms (L. Wang et al., 2018). Another epidemiological study showing variation was conducted on 48 ASD and 48 normal children from China to study the association between ASD and composition of gut microbiota. The study reveals that certain bacterial species like Firmicutes, Proteobacteria, and Verrucomicrobia, and higher levels of Bacteroidetes in ASD patients. Thus modulating the composition of microbiota can be helpful in treating ASD (Zou et al., 2020). 3 studies were conducted to check the link between gut and autism related biomarker, an investigation conducted on 64 children with ASD showed that vitamin A intervention for 6 months not only changes the level of certain bacteria it also increases plasma retinol, acid-related orphan receptor alpha RORA, mRNA levels and CD38. All of these are related to autism. All these changes indicate a link between vitamin A, gut microbiota and autism related biomarkers, but the direct link between them is still unclear (J. Liu et al., 2017). Likewise, A study was conducted to study the stool IgA levels in both ASD and healthy children. Results showed a higher IgA level in ASD than in normal individuals suggesting abnormalities of gut immune system in ASD patients, possibly providing a biomarker for ASDs. More findings are still needed to confirm these results (Zhou et al., 2018). Supporting above studies an epidemiological study was conducted on 127 children to compare the gut microbiota in children with autism spectrum disorder (ASD) to healthy controls. Children with ASD had more diverse gut microbiota and a distinct microbial structure. Specific microbial markers were identified, correlating with ASD severity, suggesting a potential diagnostic tool. These findings imply the association between gut microbiota and ASD and revealed that modulating gut microbiota can be a novel therapeutic strategy for managing ASD (Ding et al., 2020).
- Studies in autism mouse models
3 studies were performed on mouse to investigate the strong correlation between gut microbiota and brain and how microbiome alteration can cause a positive effect on autism symptoms. The author in his experimental study reported that to study the relation between autism like behavior and gut microbiota a mouse with autism was used. When the mouse was given valproic acid (VPA) during pregnancy it had similar results as were seen in human ASD studies as it affected the gut microbiota in the offspring. Increase levels of butyrate in the gut and changes in social behavior have also been observed suggesting a link between autism like behaviors and gut microbiota (De Theije et al., 2014). Another study on a maternal immune activation (S. Liu et al.) mouse model revealed that when a healthy bacterium called Bacteroides fragilis was given to MIA it not only recovered the gut functioning but also improved mice communication made it less anxious and reduced its stereotypical behaviour. Furthermore, Bacteroides fragilis also had a bad effect on metabolites of MIA offspring that are present in individuals of autism. All these findings suggest a potential treatment for both gut and ASD by showing an association between gut microbiome and conditions in autism individuals. (Hsiao et al., 2013). Likewise, Study was conducted between both male and female BTBR mice with autism and control C57 mice to examine gut microbiota, immune feature, and different psychological behavior. BTBR mice showed changes in behavior, immune responses, gut permeability increase and gut dysbiosis when compared with C57 mice. Significantly a correlation between sex specific variations in these features and a bacteria genus like Bacteroides and Sutterella were observed (Coretti et al., 2017) backing up the above studies a research was conducted on mouse model of autism BTBR mice to examine the gut microbiota and gut-brain axis. Findings found that certain bacteria such as Bifidobacterium and Blautia were reduced in BTBR mice, this reduction caused GI problems, behavioral problems and problems in bile acids and tryptophan metabolism in intestine, suggesting a link between gut microbiota and ASD and providing specific bacterial targets for treatments. (Golubeva et al., 2017). Figure 2 represents the schematic diagram of the above results.

Figure 2: ASD association with gut microbiota and potential treatments


 Anil Batta*
Anil Batta*


 10.5281/zenodo.13835680
10.5281/zenodo.13835680