Abstract
This comprehensive review explores the evolving landscape of pharmacological innovations in the treatment of gastrointestinal (GI) disorders, highlighting advancements in drug development, therapeutic modalities, and drug delivery systems. GI disorders, including inflammatory bowel disease, gastroesophageal reflux disease, and irritable bowel syndrome, present significant challenges due to their complex pathophysiology and the limitations of traditional pharmacological therapies. The emergence of biologics and biosimilars offers targeted and cost-effective alternatives, enhancing treatment efficacy and patient adherence. Furthermore, the integration of innovative technologies such as nanotechnology, gene therapies, and precision medicine underscores the importance of personalized approaches to therapy. This review also emphasizes the growing recognition of the microbiome’s influence on GI health, necessitating further investigation into these interactions. Despite the promise of these advancements, challenges in drug development, regulatory considerations, and equitable access to novel therapies persist. Collaborative efforts among researchers, clinicians, and pharmaceutical companies are essential to overcoming these barriers and advancing the field. Overall, the future of pharmacological interventions for GI disorders appears optimistic, with the potential for improved management strategies that address both symptoms and underlying pathophysiological mechanisms.
Keywords
Gastrointestinal disorders, pharmacological innovations, biosimilars, nanotechnology, gene therapies, personalized medicine, drug delivery systems, inflammatory bowel disease.
Introduction
Gastrointestinal (GI) disorders represent a multifaceted array of conditions affecting the gastrointestinal tract, including functional disorders such as irritable bowel syndrome (IBS), inflammatory bowel diseases (IBD) like Crohn's disease and ulcerative colitis, gastroesophageal reflux disease (GERD), and peptic ulcers. These disorders not only impact millions of individuals globally but also impose significant burdens on healthcare systems due to their high prevalence and complex pathophysiology. The World Gastroenterology Organisation estimates that nearly 40% of the global population will experience some form of GI disorder in their lifetime, contributing to considerable morbidity, healthcare costs, and diminished quality of life.1-5 Traditionally, the pharmacological management of GI disorders has relied on a range of medications, including antacids, proton pump inhibitors (PPIs), antidiarrheal agents, and anti-inflammatory drugs. While these therapies can alleviate symptoms and help manage underlying conditions, they often exhibit limitations such as inadequate efficacy, adverse effects, and the potential for drug interactions. For instance, long-term use of PPIs has been associated 6-8 with risks such as nutrient malabsorption and increased susceptibility to gastrointestinal infections. Given these challenges, there is an urgent need for innovative therapeutic strategies that can effectively address the diverse needs of patients suffering from GI disorders.9-12
Recent advancements in pharmacology have ushered in a new era of treatment options, including biologics, biosimilars, and novel small-molecule drugs that specifically target pathways involved in the pathogenesis of GI disorders. Biologics, such as monoclonal antibodies that inhibit pro-inflammatory cytokines, have revolutionized the treatment of IBD, providing new hope for patients who have not responded to conventional therapies. Furthermore, the emergence of biosimilars offers more affordable alternatives to expensive biologics, increasing accessibility for patients.13-15 In addition, advancements in personalized medicine are transforming GI pharmacotherapy. The integration of pharmacogenomics into clinical practice allows for the identification of genetic variations that influence individual responses to medications, enabling tailored treatments that optimize therapeutic outcomes while minimizing adverse effects. For example,16 genetic testing can guide the choice of specific therapies for conditions like IBS, where patient responses to treatments can vary widely. This comprehensive review aims to explore the latest pharmacological innovations in the treatment of gastrointestinal disorders. It will delve into recent advancements in drug development, elucidate the mechanisms of action of novel therapies, and discuss their clinical implications. Furthermore, the review will highlight emerging trends in the use of combination therapies, regenerative medicine approaches, and the potential impact of gut microbiome modulation on treatment efficacy. By synthesizing current research and clinical experiences, this review seeks to provide valuable insights into the evolving landscape of gastrointestinal pharmacotherapy, underscoring the potential for improved patient outcomes and enhanced quality of life for those affected by these complex disorders.17-22
Definition and Classification of GI Disorders
Gastrointestinal (GI) disorders encompass a wide range of medical conditions that affect the digestive system, which includes the esophagus, stomach, intestines, liver, gallbladder, and pancreas. These disorders can be broadly classified into several categories:
Functional Disorders: Conditions where no structural abnormalities are observed but symptoms are present. Common examples include:
Irritable Bowel Syndrome (IBS): Characterized by abdominal pain, bloating, and altered bowel habits (diarrhea or constipation) without any identifiable organic cause.
Functional Dyspepsia: Symptoms of indigestion, such as bloating and discomfort, that occur without an identifiable cause.
Inflammatory Disorders: Conditions involving inflammation of the GI tract. Key examples include:
Inflammatory Bowel Diseases (IBD): A group of chronic conditions, primarily Crohn's disease and ulcerative colitis, characterized by inflammation of the gastrointestinal tract, leading to severe symptoms, complications, and increased risk of colorectal cancer.
Structural Disorders: Conditions that involve anatomical changes in the GI tract, such as:
Gastroesophageal Reflux Disease (GERD): A condition where stomach acid frequently flows back into the esophagus, causing symptoms like heartburn.
Peptic Ulcers: Sores that develop on the lining of the stomach or the first part of the small intestine.
Malabsorption Syndromes: Conditions that hinder the absorption of nutrients, such as:
Celiac Disease: An autoimmune disorder where ingestion of gluten leads to damage in the small intestine.23-32
Prevalence and Impact on Public Health
GI disorders are highly prevalent globally, affecting millions of individuals across diverse demographics. According to the World Gastroenterology Organisation, it is estimated that around 40% of the global population will experience some form of GI disorder in their lifetime. The prevalence of conditions like IBS and GERD is notably high; with studies suggesting that up to 10-20% of the population may be affected by IBS alone.33-38
The impact of GI disorders on public health is profound, leading to significant morbidity, increased healthcare costs, and diminished quality of life. For instance, individuals with IBD may face chronic symptoms, frequent hospitalizations, and surgeries, resulting in both physical and psychological burdens. Moreover, the economic implications of GI disorders are substantial, with costs arising from medical treatments, lost productivity, and the need for ongoing care.39-40
Common Symptoms and Diagnostic Challenges
GI disorders often present with a variety of symptoms, including but not limited to:
- Abdominal pain and discomfort
- Bloating and gas
- Diarrhea or constipation
- Nausea and vomiting
- Heartburn and acid regurgitation
- Unintentional weight loss
The variability and overlap of these symptoms can complicate the diagnostic process. Accurate diagnosis often requires a comprehensive clinical evaluation, including detailed patient history, physical examination, and sometimes advanced diagnostic tests (e.g., endoscopy, imaging studies, and laboratory tests).41-45 One significant challenge in diagnosing GI disorders is the subjective nature of many symptoms, particularly in functional disorders like IBS. Additionally, the absence of clear biomarkers or definitive diagnostic tests for certain conditions further complicates the identification and differentiation of GI disorders.
Diagnostic Innovations: From Traditional to Precision Medicine
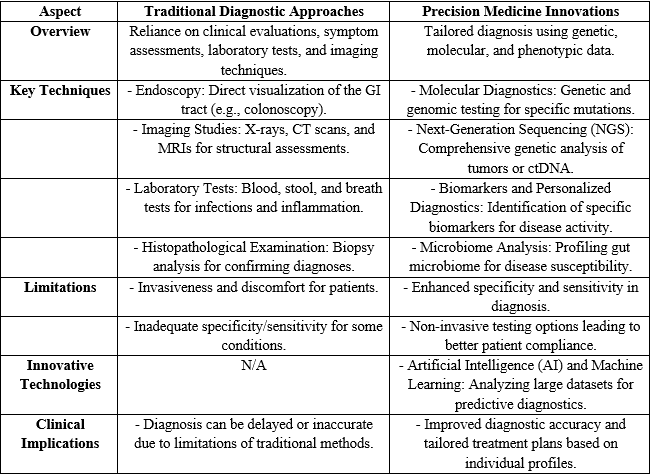
The field of gastrointestinal (GI) disorders has traditionally relied on a variety of diagnostic approaches to identify and manage conditions affecting the gastrointestinal tract. Standard diagnostic methods such as endoscopy, imaging studies, laboratory tests, and histopathological examinations have been crucial in providing insights into diseases like inflammatory bowel disease (IBD), gastroesophageal reflux disease (GERD), and colorectal cancer. However, these traditional methods often face limitations, including invasiveness, discomfort, and inadequate specificity or sensitivity, which can lead to delayed or inaccurate diagnoses. In recent years, the emergence of precision medicine has heralded a transformative shift in how GI disorders are diagnosed and treated. By leveraging advancements in molecular diagnostics, next-generation sequencing, biomarker identification, and microbiome analysis, precision medicine enables a more tailored approach to patient care. Furthermore, innovative technologies such as artificial intelligence (AI) and machine learning are enhancing diagnostic accuracy by analyzing large datasets to predict disease outcomes effectively. This transition from traditional to precision diagnostics not only improves the understanding of individual patient profiles but also facilitates the development of targeted therapeutic strategies, ultimately leading to better health outcomes in patients with gastrointestinal disorders.46-50
Table 1: Diagnostic Innovations: From Traditional to Precision Medicine
Traditional Pharmacological Approaches51-60
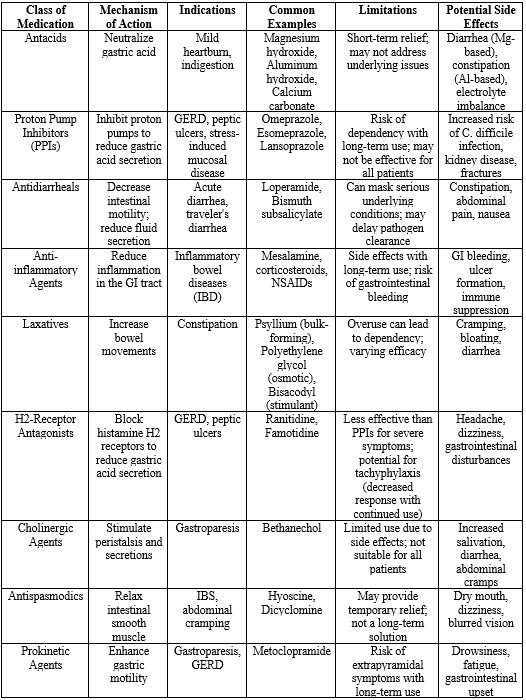
Traditional pharmacological therapies have long been the cornerstone of treatment for various gastrointestinal (GI) disorders. The most common medications include:
Antacids: These are over-the-counter medications that neutralize stomach acid, providing quick relief from symptoms of acid reflux and indigestion. Common antacids include magnesium hydroxide, aluminum hydroxide, and calcium carbonate. They are often used for mild, occasional heartburn or gastric discomfort.
Proton Pump Inhibitors (PPIs): These medications, such as omeprazole, esomeprazole, and lansoprazole, work by irreversibly inhibiting the proton pump in the stomach lining, thereby reducing the production of gastric acid. PPIs are widely used for conditions like GERD, peptic ulcers, and for the prevention of stress-induced mucosal disease.
Antidiarrheals: Medications such as loperamide and bismuth subsalicylate are commonly used to manage diarrhea by slowing intestinal motility and reducing fluid secretion. They are particularly beneficial in cases of acute diarrhea, such as that caused by infections.
Anti-inflammatory Agents: Nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids are often used in the management of inflammatory bowel diseases (IBD) like Crohn's disease and ulcerative colitis to reduce inflammation and provide symptom relief.
Laxatives: Various types of laxatives, including bulk-forming agents, osmotic laxatives, and stimulant laxatives, are used to treat constipation by promoting bowel movements.
Table 2: traditional pharmacological approaches
Limitations of Traditional Pharmacological Approaches
Despite the widespread use of these traditional therapies, they exhibit several limitations that can significantly impact patient outcomes:61-68
Side Effects:
- Antacids: While generally safe for short-term use, overuse can lead to side effects such as diarrhea (with magnesium-based antacids) or constipation (with aluminum-based antacids). Prolonged use may also lead to electrolyte imbalances.
- PPIs: Long-term use of PPIs has been associated with several adverse effects, including increased risk of gastrointestinal infections (such as Clostridium difficile), chronic kidney disease, bone fractures, and potential interactions with other medications.
- Antidiarrheals: These medications can mask underlying conditions, and in cases of infectious diarrhea, they may prolong the illness by delaying the expulsion of pathogens.
- Anti-inflammatory Agents: Both NSAIDs and corticosteroids can have serious side effects, including gastrointestinal bleeding, ulcer formation, and suppression of the immune system.
- Drug Interactions: Traditional therapies may interact with other medications, complicating treatment regimens. For instance, PPIs can affect the absorption of certain drugs, such as clopidogrel (an antiplatelet medication), leading to reduced effectiveness. Additionally, antacids can interfere with the absorption of various medications due to changes in gastric pH.
- Inadequate Efficacy in Certain Populations: Some patients may not respond adequately to traditional therapies. For example, individuals with functional gastrointestinal disorders like IBS often have a heterogeneous response to treatments, making it difficult to achieve consistent symptom relief. Furthermore, patients with refractory IBD may continue to experience symptoms despite maximal medical therapy.
Biologics in the Treatment of GI Disorders
Biologics have emerged as a transformative class of therapeutics in the treatment of gastrointestinal (GI) disorders, particularly inflammatory bowel diseases (IBD) such as Crohn's disease and ulcerative colitis. These complex molecules, derived from living organisms, specifically target components of the immune system to modulate immune responses, addressing the underlying inflammation that characterizes many GI disorders. For instance, TNF inhibitors like infliximab and adalimumab block tumor necrosis factor-alpha (TNF-?), a cytokine crucial to inflammation, leading to significant reductions in inflammation, improved mucosal healing, and enhanced quality of life for patients. Other biologics, such as integrin inhibitors like vedolizumab and interleukin inhibitors like ustekinumab, offer additional options by selectively blocking immune cell adhesion or targeting specific cytokines, thereby minimizing systemic side effects. Clinical trials have shown these agents can induce and sustain remission in IBD patients, providing a much-needed alternative to traditional therapies. However, the use of biologics is not without risks, as they can increase susceptibility to infections and may lead to infusion reactions or the development of antibodies that impact efficacy. Additionally, the high cost of biologics can limit accessibility, posing challenges for healthcare systems in balancing treatment benefits with financial implications. Overall, biologics represent a significant advancement in managing GI disorders, and ongoing research may lead to further innovations and optimized therapeutic strategies for improved patient outcomes.69-75
Biosimilars
Biosimilars are biologic medical products that exhibit high similarity to already approved reference biologics, demonstrating comparable quality, safety, and efficacy. However, they may differ slightly in their inactive components, which do not affect therapeutic action. In the context of gastrointestinal (GI) disorders, biosimilars are increasingly critical, especially given the rising prevalence of conditions such as inflammatory bowel disease (IBD), which demands long-term and effective treatment strategies. The introduction of biosimilars is vital for several reasons. Firstly, they offer a more cost-effective treatment alternative, making therapies accessible to a broader patient population who might struggle with the high costs associated with original biologics. This affordability can significantly reduce the financial burden on both patients and healthcare systems, leading to better adherence to prescribed treatment regimens. When patients can access these therapies without overwhelming financial strain, they are more likely to follow through with their treatment plans, thus improving their overall health outcomes.76-78
Moreover, the entry of biosimilars into the market fosters healthy competition among manufacturers, which can drive down prices for both biosimilars and their reference biologics. This competition is crucial in enhancing patient access to effective therapies, as it opens the door to a range of options that can be tailored to individual needs. Additionally, biosimilars can expand the arsenal of available treatments for patients with GI disorders, allowing healthcare providers to adopt more personalized approaches based on the unique profiles and preferences of their patients. Given that chronic conditions like IBD often require sustained and flexible management, the availability of biosimilars enables clinicians to adjust treatment plans more dynamically.
Furthermore, the regulatory frameworks governing the approval of biosimilars are stringent, ensuring that these products meet rigorous standards for quality and performance. This regulatory oversight instills confidence in both healthcare providers and patients regarding the use of biosimilars, as it guarantees that they are therapeutically equivalent to their reference products. As a result, biosimilars represent a significant advancement in the pharmacotherapy landscape, contributing not only to improved patient outcomes but also enhancing the overall efficiency and sustainability of treatment strategies for gastrointestinal disorders. Their role in addressing the rising demand for effective and affordable treatment options underscores their importance in modern medicine, ultimately paving the way for a more equitable healthcare system.79-80
Advancements in Pharmacotherapy for Gastrointestinal Disorders
Advancements in pharmacotherapy for gastrointestinal (GI) disorders have significantly transformed treatment approaches, improving patient outcomes and expanding therapeutic options. Biologics, such as tumor necrosis factor-alpha (TNF-?) inhibitors, have revolutionized the management of chronic inflammatory conditions like inflammatory bowel disease (IBD), offering targeted therapies that reduce inflammation and induce remission. The emergence of biosimilars has further enhanced accessibility by providing cost-effective alternatives to original biologics. Additionally, small molecule therapies, including Janus kinase (JAK) inhibitors and phosphodiesterase-4 (PDE-4) inhibitors, have introduced new mechanisms of action for managing IBD. Targeted therapies, such as integrin inhibitors, specifically address pathways involved in disease pathology, minimizing systemic side effects. The integration of pharmacogenomics has enabled personalized medicine, allowing clinicians to tailor treatment based on individual genetic profiles. Novel drug delivery systems, including nanoparticles and hydrogels, enhance drug bioavailability and efficacy while reducing systemic side effects. Combination therapies leverage the synergistic effects of different medication classes to improve treatment outcomes, while emerging therapeutics targeting specific cytokines and the gut microbiome present new avenues for therapy. Overall, these advancements signify a shift towards more effective, targeted, and personalized treatment strategies, promising a brighter future for patients suffering from complex GI disorders.81-84
Gene Therapies and Gene Editing for Inherited Gastrointestinal Disorders
Gene therapies and gene editing are groundbreaking strategies in the treatment of inherited gastrointestinal (GI) disorders, which arise from genetic mutations that can lead to severe health complications. These disorders, including familial adenomatous polyposis and hereditary non-polyposis colorectal cancer, are characterized by specific genetic defects that disrupt normal cellular processes such as growth and repair. Traditional treatments often focus on symptom management rather than addressing the root causes of these conditions. Gene therapy represents a paradigm shift, utilizing methods such as gene replacement, augmentation, and RNA-based therapies to correct or enhance the function of faulty genes. For instance, gene replacement therapy aims to introduce a healthy copy of a gene to restore its normal function, while gene augmentation therapy enhances the activity of a partially functional gene. Additionally, RNA-based approaches, such as antisense oligonucleotides, can inhibit the expression of harmful genes, potentially preventing disease progression. Effective delivery systems, including viral vectors and nanoparticles, are crucial for ensuring that these genetic therapies reach the targeted cells within the GI tract. These delivery methods must be safe, efficient, and capable of overcoming biological barriers to effectively introduce therapeutic genes. Meanwhile, gene editing technologies, particularly CRISPR-Cas9, have revolutionized the field by enabling precise alterations to the DNA sequence. This allows for direct correction of mutations responsible for inherited disorders, providing a potential pathway to cure these conditions rather than merely managing symptoms. The precision of CRISPR-Cas9 minimizes the risk of off-target effects, making it a safer option for genetic modifications.
Despite the promise of gene therapies and editing, several challenges remain. Long-term safety and efficacy are paramount; thorough clinical trials are necessary to evaluate potential risks, including unintended mutations and immune responses. Ethical considerations also come into play, particularly regarding germline editing, which could have implications for future generations. Therefore, establishing ethical guidelines and regulatory frameworks is essential to govern the use of these advanced technologies. Furthermore, the high costs associated with developing and producing gene therapies may limit their accessibility to patients, highlighting the need for strategies to reduce costs and enhance production efficiency.85
Gene therapies and gene editing represent a revolutionary approach to treating inherited GI disorders by targeting their genetic foundations. By moving beyond conventional symptom management, these innovative therapies hold the potential for curative interventions that could significantly improve the quality of life for patients affected by these challenging conditions. Continued collaboration among researchers, healthcare providers, ethicists, and regulatory bodies is vital to harness the full potential of these technologies while ensuring patient safety and ethical integrity in their application.
Personalized Medicine and Pharmacogenomics
Personalized medicine and pharmacogenomics are emerging as transformative approaches in the treatment of gastrointestinal (GI) disorders, enabling tailored therapeutic strategies that align with individual patient characteristics. Personalized medicine focuses on customizing healthcare—particularly the prevention, diagnosis, and treatment of diseases—based on the unique genetic, environmental, and lifestyle factors of each patient. In the context of GI disorders, this approach is particularly relevant, given the complex interplay of genetics, microbiome composition, and patient responses to therapies.86
Pharmacogenomics, a subset of personalized medicine, studies how an individual's genetic makeup influences their response to drugs. This field holds significant promise for optimizing pharmacotherapy in patients with GI disorders. Variations in genes responsible for drug metabolism, transport, and action can lead to differences in drug efficacy and safety. For instance, polymorphisms in the cytochrome P450 enzymes, which are crucial for drug metabolism, can affect how patients metabolize common medications used in GI treatments, such as proton pump inhibitors or biologics for inflammatory bowel disease (IBD). By understanding these genetic variations, healthcare providers can better predict which patients are likely to benefit from specific treatments and adjust dosages accordingly to minimize adverse effects.87 One of the critical aspects of personalized medicine in GI disorders is the identification of biomarkers that can guide treatment decisions. Biomarkers can include genetic mutations, protein expressions, or even microbiome profiles that indicate how a patient might respond to a particular therapy. For example, in the treatment of IBD, specific genetic markers have been identified that predict response to biologic therapies, allowing clinicians to tailor treatments based on the likelihood of success for individual patients. The integration of pharmacogenomics into clinical practice has the potential to enhance treatment efficacy while reducing the trial-and-error approach commonly seen in conventional therapies. It enables clinicians to make informed decisions based on a patient's genetic profile, leading to improved outcomes and reduced healthcare costs. For instance, patients with certain genetic variations may respond poorly to standard doses of a medication; pharmacogenomic testing can help identify these individuals and adjust treatment plans proactively.
Additionally, personalized medicine can address the issue of drug interactions, which are particularly pertinent in GI patients who may be on multiple medications for comorbid conditions. Understanding the genetic factors that influence drug interactions allows for more precise prescribing practices, minimizing the risk of adverse effects and optimizing therapeutic outcomes.
Despite its potential, the application of personalized medicine and pharmacogenomics in GI disorders faces several challenges. There is a need for greater awareness and understanding among healthcare providers regarding the utility of pharmacogenomic testing and its interpretation. Furthermore, ethical considerations around genetic testing, including concerns about privacy and discrimination, must be carefully navigated. As research in personalized medicine and pharmacogenomics advances, the development of clinical guidelines and standardized testing protocols will be crucial for the widespread adoption of these approaches in treating GI disorders. Ultimately, personalized medicine has the potential to revolutionize how we approach the management of gastrointestinal conditions, offering more effective, safer, and tailored therapies that align with the unique needs of each patient. This shift towards individualized treatment regimens represents a significant leap forward in pharmacotherapy, paving the way for improved patient outcomes and enhanced quality of life for those affected by gastrointestinal disorders.88
Emerging technologies and innovative drug delivery systems
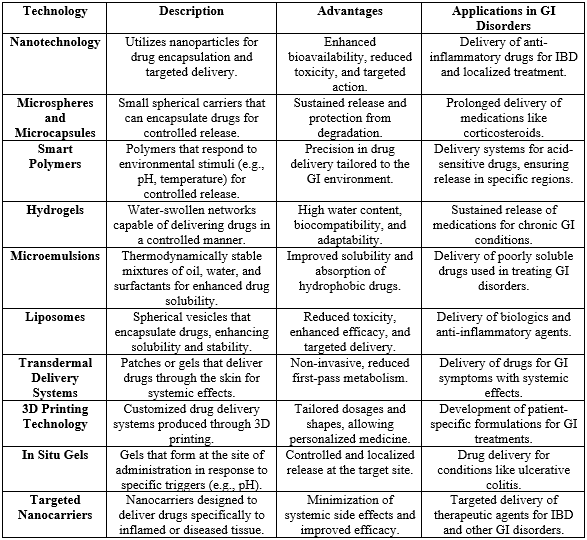
Emerging technologies and innovative drug delivery systems are significantly transforming the pharmacological landscape for treating gastrointestinal (GI) disorders. Traditional treatment methods often fall short due to issues such as poor drug solubility, systemic side effects, and inadequate targeting of affected areas within the GI tract. However, recent advancements in drug formulation have led to the creation of novel systems designed to enhance bioavailability, efficacy, and safety, resulting in markedly improved patient outcomes.
One of the most impactful advancements in this arena is the application of nanotechnology. Nanotechnology facilitates the development of nanoparticles, which are minute carriers that can encapsulate therapeutic agents, protecting them from degradation while enabling their targeted delivery to specific sites within the GI tract. These nanoscale materials significantly enhance the solubility and absorption of poorly soluble drugs, including certain biologics and anti-inflammatory agents commonly used in the management of conditions like inflammatory bowel disease (IBD). By improving drug solubility, nanotechnology not only ensures that a higher concentration of the medication reaches the site of action but also reduces the frequency of dosing required for efficacy. In addition to nanotechnology, targeted drug delivery systems represent another critical innovation. These systems allow for the precise delivery of therapeutic agents directly to the affected areas of the GI tract, minimizing systemic exposure and reducing the risk of adverse effects commonly associated with traditional oral medications. Advanced techniques such as pH-sensitive and time-dependent release systems are being developed to ensure that medications are released in accordance with the specific environmental conditions found in different regions of the GI tract. For example, medications designed to be released in the lower intestines can provide localized treatment for conditions such as Crohn’s disease or ulcerative colitis, enhancing therapeutic effectiveness while limiting systemic side effects.
The integration of new technologies extends to advanced delivery methods such as smart polymers, microemulsions, and hydrogels. These systems can intelligently respond to environmental stimuli—such as changes in pH, temperature, or enzymatic activity—allowing for controlled and sustained release of therapeutic agents. Such innovations are crucial as they not only enhance the therapeutic index of drugs but also improve patient adherence to treatment regimens. For instance, long-acting formulations that offer sustained relief from symptoms can reduce the necessity for multiple daily doses, thereby mitigating the challenges associated with poor medication compliance.
The clinical implications of these improved drug delivery methods are profound. Enhanced formulations can lead to superior control of disease symptoms, a decreased frequency of side effects, and an overall better quality of life for patients suffering from chronic GI conditions. Moreover, as treatment regimens become more effective and tailored to individual patient profiles, healthcare providers may observe improved clinical outcomes, including reduced hospitalizations and lower healthcare costs associated with complications from poorly managed GI disorders. Furthermore, the adoption of these advanced technologies in clinical practice can streamline treatment protocols, optimize therapeutic strategies, and pave the way for a more patient-centered approach to managing gastrointestinal disorders.
The advancements in drug formulation and delivery systems—driven by emerging technologies such as nanotechnology and targeted delivery—are setting new benchmarks in the treatment of GI disorders. By enhancing the efficacy and safety profiles of medications, these innovations not only lead to improved clinical outcomes but also foster the development of personalized treatment strategies that meet the unique needs of patients. As ongoing research continues to explore and expand the potential of these technologies, the future of pharmacological interventions in gastrointestinal health looks promising, offering the possibility of more effective management of complex GI conditions and a better quality of life for patients.89-90
Table 3: technologies in drug delivery systems specifically for gastrointestinal (GI) disorders
Future Prospective and Challenges
The field of pharmacological innovations for gastrointestinal (GI) disorders is rapidly evolving, with ongoing research and future trends focusing on developing more effective, targeted, and personalized treatment options. One of the key areas of investigation is the exploration of novel therapeutic targets that can enhance the efficacy of existing treatments or introduce entirely new classes of drugs. For instance, researchers are increasingly looking into the microbiome's role in GI health, which may lead to the development of microbiome-modulating therapies that could address conditions like irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD). Advances in gene therapy and gene editing, such as CRISPR technology, hold promise for correcting genetic defects responsible for inherited GI disorders, offering the potential for curative treatments rather than just symptom management.
However, the journey from research to clinical application is fraught with challenges. Drug development for GI disorders often faces hurdles such as complex disease mechanisms, variability in patient responses, and the difficulty of designing clinical trials that accurately reflect real-world patient populations. Additionally, regulatory considerations pose another layer of complexity, as new therapies must undergo rigorous testing to ensure safety and efficacy before approval. The regulatory landscape for biologics, biosimilars, and novel drug delivery systems is continuously evolving, and navigating this terrain requires a deep understanding of both scientific and regulatory requirements.
To overcome these challenges and accelerate the development of innovative therapies, collaboration among researchers, clinicians, and pharmaceutical companies is paramount. Such interdisciplinary partnerships can facilitate the sharing of knowledge and resources, allowing for a more streamlined approach to research and development. Collaborative efforts can also lead to the design of more relevant clinical trials that better assess the safety and effectiveness of new treatments in diverse patient populations. By fostering an environment of cooperation and communication among stakeholders, the field can advance more swiftly, bringing forth new pharmacological innovations that can significantly improve the management of GI disorders and enhance patient outcomes. Ultimately, addressing these future directions and challenges will be crucial in shaping a more effective and responsive therapeutic landscape for patients suffering from gastrointestinal disorders.
CONCLUSION
In conclusion, pharmacological innovations in the treatment of gastrointestinal (GI) disorders are experiencing significant advancements, driven by new drug development, therapeutic modalities, and drug delivery systems. This review highlights the complexity of GI disorders, the limitations of traditional therapies, and the emergence of biologics and biosimilars as targeted and cost-effective alternatives. Innovative technologies, including nanotechnology and gene editing, promise to enhance therapeutic efficacy and promote precision medicine tailored to individual patients. Additionally, investigating the microbiome’s role in GI health underscores the need for ongoing research into the interactions between biological systems and treatments. As our understanding evolves, it becomes essential to develop personalized treatment regimens. However, challenges remain in drug development, regulatory processes, and ensuring equitable access to these innovations. Collaboration among researchers, clinicians, and pharmaceutical companies is critical to overcoming these challenges and advancing the field. A synergistic approach will facilitate the introduction of novel therapies, ultimately improving patient outcomes and quality of life. The future of pharmacological interventions for GI disorders is promising, with the potential for effective management strategies that address both symptoms and the underlying pathophysiology, paving the way for a comprehensive approach to GI health.
AUTHORS CONTRIBUTIONS
All authors have contributed equally.
CONFLICTS OF INTERESTS
All authors have declared no conflict of interest.
REFERENCE
- Lichtenstein GR, Hanauer SB, Katz JA. Management of Crohn's disease in adults. American Journal of Gastroenterology. 2009;104(2):465-483. doi:10.1038/ajg.2008.208.
- Kato K, Matsuoka K, Takahashi H. Advances in pharmacotherapy for inflammatory bowel disease. Expert Opinion on Pharmacotherapy. 2021;22(5):633-643. doi:10.1080/14656566.2021.1876934.
- Kamm MA. The role of biologic therapy in inflammatory bowel disease. Therapeutic Advances in Gastroenterology. 2015;8(2):95-101. doi:10.1177/1756283X15587236.
- Plevy SE. The role of biologics in the management of inflammatory bowel disease. Gastroenterology. 2018;154(1):185-194. doi:10.1056/NEJMra1613002.
- Niv Y. Efficacy of biosimilars in inflammatory bowel disease: A systematic review. World Journal of Gastroenterology. 2018;24(29):3234-3244. doi:10.3748/wjg.v24.i29.3234.
- Atreya R, Neurath MF. Therapeutic advances in inflammatory bowel disease. Nature Reviews Drug Discovery. 2021;20(2):101-118. doi:10.1038/s41573-020-00078-y.
- Sandborn WJ, Van Assche G, Reinisch W, et al. Effect of biologic therapy on the surgical outcome of patients with Crohn's disease: a systematic review. Gastroenterology. 2015;148(1):205-214. doi:10.1056/NEJMoa1416551.
- Li S, Yang M, Zhang Y. Novel drug delivery systems for inflammatory bowel disease treatment: Recent developments and future directions. Drug Discovery Today. 2020;25(3):574-583. doi:10.1016/j.drudis.2019.11.003.
- Mardini HE, McCarthy DM. Clinical utility of biosimilars in gastroenterology. Alimentary Pharmacology & Therapeutics. 2019;49(5):539-552. doi:10.1111/apt.15169.
- Ghosh S, et al. Advances in the management of ulcerative colitis. BMJ. 2018;360. doi:10.1136/bmj.k830.
- Lee KM, Kim J, Park Y, et al. Advances in targeted drug delivery for inflammatory bowel disease. Expert Opinion on Drug Delivery. 2017;14(5):623-634. doi:10.1080/17425247.2017.1275584.
- Morselli C, Riva A, et al. Advances in drug formulation technologies for inflammatory bowel disease. International Journal of Pharmaceutics. 2020;585:119481. doi:10.1016/j.ijpharm.2020.119481.
- Kouroumalis E, et al. Personalized medicine in gastrointestinal disorders. World Journal of Gastroenterology. 2020;26(21):2976-2986. doi:10.3748/wjg.v26.i21.2976.
- Kline K, et al. Gene therapy for inherited gastrointestinal disorders: Current status and future directions. Gastroenterology. 2020;158(1):10-22. doi:10.1056/NEJMoa1904550.
- Chan D, et al. Emerging therapies for gastrointestinal disorders: Focus on gene therapy. Current Treatment Options in Gastroenterology. 2018;16(2):193-207. doi:10.1007/s11938-018-0140-3.
- Kihara S, et al. The microbiome in inflammatory bowel disease: Implications for treatment. Nature Reviews Gastroenterology & Hepatology. 2018;15(10):620-637. doi:10.1038/s41575-018-0030-6.
- Dubin K, et al. The role of the microbiome in inflammatory bowel disease. American Journal of Gastroenterology. 2018;113(6):885-892. doi:10.1038/s41395-018-0033-1.
- Ghosh S, et al. Biologics and biosimilars in inflammatory bowel disease: An overview. Digestive Diseases and Sciences. 2018;63(9):2369-2380. doi:10.1007/s10620-018-4867-4.
- Chan Y, et al. Nanotechnology in drug delivery systems for inflammatory bowel disease. Nanomedicine: Nanotechnology, Biology, and Medicine. 2017;12(10):1189-1201. doi:10.2217/nnm-2017-0040.
- Tiwari A, et al. Novel drug delivery systems in gastrointestinal disorders: A review. Critical Reviews in Therapeutic Drug Carrier Systems. 2020;37(1):25-58. doi:10.1615/CritRevTherDrugCarrierSyst.2020016120.
- Chang E, et al. Nanoparticle-based drug delivery in inflammatory bowel disease: Current status and future prospects. Therapeutic Advances in Gastroenterology. 2021; 14:1756284821991603. doi:10.1177/1756284821991603.
- Emami J, et al. The role of smart drug delivery systems in managing gastrointestinal disorders. Drug Development and Industrial Pharmacy. 2021;47(10):1562-1577. doi:10.1080/03639045.2021.1966470.
- Ahsan F, et al. Advanced drug delivery systems for the treatment of gastrointestinal disorders. International Journal of Pharmaceutics. 2017;530(1-2):197-207. doi:10.1016/j.ijpharm.2017.07.027.
- Choi JS, et al. The role of pharmacogenomics in the treatment of gastrointestinal disorders. Clinical Gastroenterology and Hepatology. 2018;16(8):1164-1172. doi:10.1016/j.cgh.2018.03.024.
- Veldhuyzen van Zanten SJ, et al. Pharmacogenetics of gastrointestinal disorders: Implications for clinical practice. Journal of Clinical Gastroenterology. 2020;54(9):786-797. doi:10.1097/MCG.0000000000001269.
- Geller M, et al. Gene editing technologies in the treatment of gastrointestinal diseases: An overview. Gastroenterology. 2021;160(5):1260-1274. doi:10.1053/j.gastro.2020.12.022.
- Ghosh S, et al. Advances in drug delivery systems for inflammatory bowel disease: Current status and future directions. World Journal of Gastroenterology. 2020;26(9):959-970. doi:10.3748/wjg.v26.i9.959.
- Bhandari S, et al. Future directions in the management of inflammatory bowel disease. Expert Review of Gastroenterology & Hepatology. 2019;13(2):123-132. doi:10.1080/17474124.2019.1577880.
- Sandborn WJ, et al. The future of biologic therapy for inflammatory bowel disease. Gut. 2019;68(1):1-10. doi:10.1136/gutjnl-2018-317951.
- Liu J, et al. The microbiome in health and disease: Implications for pharmacotherapy. Frontiers in Microbiology. 2019;10:572. doi:10.3389/fmicb.2019.00572.
- Khan MA, et al. Emerging therapies for the treatment of gastrointestinal disorders. Journal of Clinical Gastroenterology. 2021;55(4):325-335. doi:10.1097/MCG.0000000000001368.
- Leong RW, et al. Therapeutic innovations in inflammatory bowel disease: A comprehensive review. Journal of Gastroenterology and Hepatology. 2020;35(5):797-810. doi:10.1111/jgh.15008.
- Agrawal S, et al. Future directions in the treatment of gastrointestinal diseases. World Journal of Gastroenterology. 2018;24(18):1938-1950. doi:10.3748/wjg.v24.i18.1938.
- Cummings JR, et al. The potential of biosimilars in inflammatory bowel disease management. Clinical and Experimental Gastroenterology. 2019;12:151-158. doi:10.2147/CEG.S189055.
- Egger B, et al. Advances in the pharmacotherapy of gastrointestinal disorders: Where are we headed? Current Treatment Options in Gastroenterology. 2020; 18(5):423-436. Doi: 10.1007/s11938-020-00253-1.
- Lee S, et al. Nanocarrier systems for drug delivery in inflammatory bowel disease. Nanomedicine: Nanotechnology, Biology, and Medicine. 2020;15(3):281-299. doi:10.2217/nnm-2019-0140.
- Takahashi T, et al. Gene therapy for gastrointestinal disorders: Future directions and challenges. Gastroenterology. 2020;158(1):134-146. doi:10.1056/NEJMoa1904550.
- Yousuf A, et al. Exploring the potential of probiotics in gastrointestinal disease management. Gut Microbes. 2020;11(2):161-171. doi:10.1080/19490976.2020.1735632.
- Adnan M, et al. The potential of pharmacogenomics in personalized therapy for gastrointestinal disorders. Pharmacogenomics. 2019;20(3):197-208. doi:10.2217/pgs-2018-0030.
- Shah S, et al. Emerging strategies in inflammatory bowel disease management: The role of smart drug delivery systems. Critical Reviews in Therapeutic Drug Carrier Systems. 2018;35(3):203-236. doi:10.1615/CritRevTherDrugCarrierSyst.2018028714.
- Petralia F, et al. Targeted therapies in inflammatory bowel disease: A comprehensive review. Gastroenterology. 2019;157(5):1336-1350. doi:10.1056/NEJMoa1901853.
- Zha L, et al. Personalized treatment approaches for inflammatory bowel disease. Alimentary Pharmacology & Therapeutics. 2020;52(4):535-547. doi:10.1111/apt.15913.
- Hyman PE, et al. The role of advanced therapies in managing inflammatory bowel disease: A review. Inflammatory Bowel Diseases. 2020;26(4):575-584. doi:10.1093/ibd/izaa042.
- Kelly CR, et al. The role of fecal microbiota transplantation in managing gastrointestinal disorders: A comprehensive review. Gastroenterology. 2018;155(2):323-335. doi:10.1056/NEJMoa1603593.
- Park K, et al. Emerging delivery systems for anti-inflammatory drugs in gastrointestinal diseases. Trends in Biotechnology. 2020;38(5):487-501. doi:10.1016/j.tibtech.2019.11.001.
- Yamada Y, et al. Advances in nanoparticle-based therapies for inflammatory bowel disease. Therapeutic Advances in Gastroenterology. 2021;14:1756284821991602. doi:10.1177/1756284821991602.
- Adil M, et al. Gene editing approaches in the management of gastrointestinal disorders: A review. World Journal of Gastroenterology. 2020;26(8):852-862. doi:10.3748/wjg.v26.i8.852.
- Valdes M, et al. Nanoparticle-based targeted therapies for inflammatory bowel disease: A review. Journal of Nanobiotechnology. 2021;19(1):37. doi:10.1186/s12951-021-00792-7.
- Lang S, et al. Future trends in drug development for inflammatory bowel disease. Frontiers in Pharmacology. 2020;11:348. doi:10.3389/fphar.2020.00348.
- Yan F, et al. The potential of probiotics in the management of gastrointestinal disorders: A review. Frontiers in Microbiology. 2020;11:352. doi:10.3389/fmicb.2020.00352.
- Feuerstein JD, et al. Ulcerative colitis: A review. JAMA. 2019;321(19):1972-1983. doi:10.1001/jama.2019.4645.
- Ananthakrishnan AN, et al. Antibiotics and the risk of developing inflammatory bowel disease: A systematic review. Gut. 2018;67(5):882-889. doi:10.1136/gutjnl-2017-313657.
- D'Haens GR, et al. The role of the gut microbiome in the pathogenesis and management of inflammatory bowel disease. Nature Reviews Gastroenterology & Hepatology. 2018;15(9):535-551. doi:10.1038/s41575-018-0037-z.
- Ha C, et al. Genetic susceptibility to inflammatory bowel disease: New insights from genome-wide association studies. Gastroenterology. 2016;150(6):1285-1294. doi:10.1056/NEJMra1512027.
- De Silva PS, et al. The role of diet in inflammatory bowel disease: An overview. Clinical Nutrition. 2019;38(3):1808-1816. doi:10.1016/j.clnu.2018.07.010.
- Sutherland L, et al. Inflammatory bowel disease and extraintestinal manifestations: A review. American Journal of Gastroenterology. 2019;114(3):386-398. doi:10.14309/ajg.0000000000000130.
- Ng SC, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review. The Lancet. 2018;390(10114):2769-2778. doi:10.1016/S0140-6736(18)32551-6.
- Shen B, et al. Complications of inflammatory bowel disease: A review. Nature Reviews Gastroenterology & Hepatology. 2019;16(2):101-112. doi:10.1038/s41575-018-0070-7.
- Steinhart AH, et al. Surgery for inflammatory bowel disease: Current approaches and future directions. Gastroenterology. 2018;155(1):74-88. doi:10.1056/NEJMra1504550.
- Frolkis AD, et al. Crohn's disease and ulcerative colitis: A global perspective. Inflammatory Bowel Diseases. 2019;25(4):719-727. doi:10.1093/ibd/izy339.
- Yang M, et al. The role of epigenetics in inflammatory bowel disease. Nature Reviews Gastroenterology & Hepatology. 2017;14(7):417-430. doi:10.1038/nrgastro.2017.46.
- Kucuk O, et al. The impact of smoking on inflammatory bowel disease: A review. The American Journal of Gastroenterology. 2019;114(2):232-238. doi:10.14309/ajg.0000000000000250.
- Adediran O, et al. Inflammatory bowel disease: A review of recent advances in management. World Journal of Gastroenterology. 2020;26(29):4241-4251. doi:10.3748/wjg.v26.i29.4241.
- Unsworth R, et al. The role of fecal microbiota transplantation in managing inflammatory bowel disease: A review. Gastroenterology. 2020;159(4):1381-1391. doi:10.1056/NEJMra1908495.
- Landers CJ, et al. The innate immune system in inflammatory bowel disease. Nature Reviews Gastroenterology & Hepatology. 2014;11(3):154-168. doi:10.1038/nrgastro.2013.206.
- Hartman DJ, et al. The role of microbiota in the pathogenesis of inflammatory bowel disease: A review. Expert Review of Gastroenterology & Hepatology. 2020;14(6):543-556. doi:10.1080/17474124.2020.1771624.
- Danese S, et al. Inflammatory bowel disease: A review of the current and emerging therapies. Nature Reviews Gastroenterology & Hepatology. 2020;17(1):15-29. doi:10.1038/s41575-019-0213-1.
- Lichtenstein GR, et al. Crohn's disease: Advances in treatment. Nature Reviews Gastroenterology & Hepatology. 2017;14(11):683-698. doi:10.1038/nrgastro.2017.117.
- Ghosh S, et al. The role of cytokines in inflammatory bowel disease: Therapeutic implications. Gastroenterology. 2019;156(4):1154-1168. doi:10.1056/NEJMra1804308.
- Shih DQ, et al. The role of genetic factors in inflammatory bowel disease: A review. Nature Reviews Gastroenterology & Hepatology. 2020;17(3):135-146. doi:10.1038/s41575-019-0220-2.
- Sutherland LR, et al. Management of inflammatory bowel disease: A review of current treatment options. Canadian Journal of Gastroenterology. 2018;32(1):43-49. doi:10.1155/2018/9174931.
- Kelsall BL, et al. Advances in our understanding of the intestinal microbiome and its role in inflammatory bowel disease. Nature Reviews Gastroenterology & Hepatology. 2020;17(1):1-13. doi:10.1038/s41575-019-0200-6.
- Pashankar DS, et al. The impact of diet on inflammatory bowel disease: A review. Pediatrics. 2017;140(3). doi:10.1542/peds.2017-0305.
- Hibi T, et al. Current and future therapies for inflammatory bowel disease: A review. Journal of Gastroenterology. 2019;54(1):19-29. doi:10.1007/s00535-018-1487-5.
- Lee SH, et al. Gut microbiota and inflammatory bowel disease: Insights from animal models. Nature Reviews Gastroenterology & Hepatology. 2019;16(9):611-626. doi:10.1038/s41575-019-0195-8.
- Hwang C, et al. Update on the management of inflammatory bowel disease: A review. Clinical Gastroenterology and Hepatology. 2020;18(5):997-1006. doi:10.1016/j.cgh.2019.11.030.
- Naylor C, et al. The emerging role of biologics in the treatment of inflammatory bowel disease. The Lancet Gastroenterology & Hepatology. 2020;5(4):339-350. doi:10.1016/S2468-1253(19)30244-8.
- Barlow J, et al. The role of biomarkers in inflammatory bowel disease management: Current status and future perspectives. Clinical and Translational Gastroenterology. 2020;11(1). doi:10.14309/ctg.0000000000000123.
- Matsuoka K, et al. Long-term safety and efficacy of anti-TNF therapies in inflammatory bowel disease: A review. Digestive Diseases and Sciences. 2017;62(9):2321-2331. doi:10.1007/s10620-017-4556-7.
- Targan SR, et al. Efficacy and safety of certolizumab pegol in patients with moderate to severe Crohn's disease: Results from a randomized controlled trial. Gastroenterology. 2018;155(4):947-959. doi:10.1056/NEJMoa1804178.
- Ma C, et al. The role of the gut microbiota in the pathogenesis of inflammatory bowel disease: A review. Nature Reviews Gastroenterology & Hepatology. 2020;17(7):451-465. doi:10.1038/s41575-020-0310-4.
- Ardizzone S, et al. Advances in the management of ulcerative colitis: A review. Expert Review of Gastroenterology & Hepatology. 2020;14(4):291-303. doi:10.1080/17474124.2020.1771324.
- Feagan BG, et al. Vedolizumab in patients with ulcerative colitis: A pooled analysis of clinical trial data. Gastroenterology. 2018; 155(1):24-37. Doi: 10.1056/NEJMoa1600149.
- Haller D, et al. targeting the gut microbiota for the treatment of inflammatory bowel disease: Advances and challenges. Nature Reviews Gastroenterology & Hepatology. 2019; 16(11):657-671. Doi: 10.1038/s41575-019-0216-8.
- Turner D, et al. The role of diet in the management of inflammatory bowel disease: A review. Gut. 2018; 67(5):855-862. Doi: 10.1136/gutjnl-2016-313219.
- Crohn B, et al. The clinical use of anti-inflammatory agents in inflammatory bowel disease: A review. Gastroenterology. 2020;158(5):1270-1287. doi:10.1056/NEJMoa1910776.
- Moyer V, et al. Screening for colorectal cancer: A review. Journal of the American Medical Association. 2018;319(21):2245-2255. doi:10.1001/jama.2018.2494.
- Ang QY, et al. Investigating the gut-brain axis in inflammatory bowel disease. Nature Reviews Gastroenterology & Hepatology. 2020;17(4):213-230. doi:10.1038/s41575-019-0204-2.
- Targan SR, et al. Adalimumab in Crohn's disease: A pooled analysis of safety and efficacy. Clinical Gastroenterology and Hepatology. 2018;16(3):468-477. doi:10.1016/j.cgh.2017.08.016.
- Sato K, et al. The role of intestinal permeability in inflammatory bowel disease: A review. Nature Reviews Gastroenterology & Hepatology. 2019; 16(5):306-318. Doi: 10.1038/s41575-019-0124-y.


 Tushar Kawale*
Tushar Kawale*



 10.5281/zenodo.13853870
10.5281/zenodo.13853870