Abstract
The integration of Artificial Intelligence (AI) and Machine Learning (ML) in pharmaceutical quality assurance (QA) presents transformative opportunities for improving the accuracy, efficiency, and consistency of quality control processes. This review explores the key applications of AI/ML in QA, including data analysis, predictive modelling, automation of routine tasks, and real-time quality monitoring. By harnessing AI, pharmaceutical companies can enhance regulatory compliance, streamline documentation, and improve decision-making through intelligent decision support systems. Drug development is a time consuming, expensive, and extremely risky procedure. Up to 90% of drug concept are discard due to challenges such as toxicity safety and efficacy resulting significant loss of investor. AI's capabilities range from enhancing accuracy and minimizing error to enabling previously impossible new ideas. AI powered quality assurance framework, leveraging machine learning, computer vision, and predictive analytics to ensure unparalleled quality excellence.
Keywords
Data driven decision making, Artificial intelligence (AI), Artificial general intelligence, drug discovery, Process learning technology (PAT), clinical trials, Digital transformation, pharma marketing, Drug development
Introduction
Pharmaceutical Quality Assurance (QA) ensures that products meet the required standards of quality, safety, and efficacy. When incorporating AI and Machine Learning (ML) into pharmaceutical processes, QA principles must be maintained to ensure compliance with regulatory standards and patient safety.

Artificial intelligence:
Artificial Intelligence (AI) refers to the simulation of human intelligence in machines that are programmed to think and perform tasks like humans. Al is sometimes described as a technology that enables machines to mimic a variety of intricate human talents. The idea of Al was first put up in 1956 during a meeting organized by Marvin Minsky and John McCarthy.
Classification of AI
- Artificial Narrow Intelligence (ANI): This Al can perform specific tasks well, like facial recognition, driving cars, or playing chess, but it's limited to those tasks and lacks general human-like intelligence.
- Artificial General Intelligence (AGI): AGI is as smart as a human and can handle a wide range of tasks, learn from experience, and solve different problems, just like humans do.
- Artificial Super Intelligence (ASI): ASI is even smarter than humans and can excel in areas like advanced mathematics, painting, and scientific research, going beyond human abilities. Building of an Al planner.
- The synergy between AI and quality assurance (QA) in the pharmaceutical industry creates a powerful combination that enhances both the efficiency and accuracy of the drug production and regulatory processes. Here are some key ways this synergy is realized:
- Enhanced Data Processing and Analysis
Massive Data Handling: Pharmaceutical QA involves the collection and analysis of vast amounts of data from clinical trials, manufacturing processes, and post-market surveillance
AI, particularly machine learning (ML), can process this data faster and more efficiently than humans, uncovering hidden patterns and potential quality risks that manual methods might miss.
Real-time Monitoring: AI enables real-time monitoring and analysis of production data, ensuring that any deviations from the standard are detected early. This allows for immediate corrective actions, reducing the likelihood of defects.
- Predictive Quality Assurance
Predictive Analytics: AI can analyze historical data from manufacturing processes and equipment performance to predict potential failures or deviations in product quality before they occur. This predictive approach helps companies anticipate and prevent quality issues rather than merely responding to them after the fact.
Early Warning Systems: AI-powered systems can act as early warning tools, alerting QA teams to potential risks in product batches based on past trends, environmental factors, or even minor variances in production.
- Automated Quality Control
Inspection and Testing: AI can automate repetitive quality control tasks such as visual inspections of products, detecting defects that are too small for human eyes to catch, and ensuring consistent quality across batches.
Process Optimization: AI-driven systems can optimize manufacturing processes in real-time, ensuring they remain within regulatory and quality guidelines, reducing variability, and enhancing product consistency.
- Improved Decision Support
AI-Enhanced Decision Making: AI can provide QA teams with advanced decision-support tools, helping them make more informed decisions based on data-driven insights. These systems analyze large datasets and provide recommendations on product release, risk management, and compliance actions, increasing confidence in decision-making processes.
Risk-Based QA: AI models can help implement a risk-based approach to QA by prioritizing resources and attention to areas of highest risk, optimizing the allocation of QA efforts, and improving overall compliance efficiency.
- Compliance and Regulatory Support
Automated Compliance Checks: AI can help streamline the regulatory compliance process by automatically checking records, ensuring that all production steps comply with Good Manufacturing Practices (GMP), and alerting QA teams to any deviations.
Audit Readiness: AI systems can help maintain a continuous audit trail by organizing and analysing documentation for regulatory audits, improving traceability and reducing the manual burden on QA teams.
- Reduction in Human Error
Automation of Manual Tasks: AI reduces the reliance on manual, repetitive tasks in QA, such as data entry, documentation, and routine checks, minimizing human error.
Consistency and Accuracy: Unlike humans, AI-driven QA processes are not subject to fatigue or bias, ensuring consistent application of quality checks across all products and batches.
- Faster Time-to-Market
Speed and Efficiency: The ability of AI to quickly analyze data, predict outcomes, and automate tasks significantly reduces the time needed for QA reviews. This results in faster product release without compromising safety or efficacy.
Innovation in Drug Development: By integrating AI in QA, pharmaceutical companies can accelerate drug development processes, as quality checks are optimized and become more seamless across stages of production.
- Cost Efficiency
Lower Operational Costs: AI-driven automation reduces the need for large manual QA teams, cutting down on labor costs while maintaining high levels of accuracy and quality.
Fewer Product Recalls: By identifying potential quality issues early and ensuring tighter control over production processes, AI helps reduce the costs associated with product recalls or quality failures.
- Adaptive Learning and Continuous Improvement
Self-Learning Algorithms: AI systems can learn and improve over time, adapting to new data inputs and refining their quality checks, leading to continuous process improvements. Feedback Loops: AI can integrate feedback from QA results back into the manufacturing process, enabling dynamic adjustments that enhance product quality over time. Machine learning:
Machine learning (ML) in pharmaceutical quality assurance (QA) is a game changer, helping companies ensure that drugs meet strict quality standards more efficiently and accurately. Here’s how machine learning is applied in QA:
- Predictive Analytics:
Machine learning algorithms can analyze vast amounts of historical data from manufacturing processes to predict potential quality issues before they occur. This helps in preventing defects or irregularities in drug production, reducing waste and improving overall efficiency.
- Real-Time Monitoring:
Machine learning systems can monitor production in real-time, analysing data from sensors and machinery to detect any deviations from expected parameters. If a batch is showing signs of a problem, the system can flag it immediately, allowing for quick corrective action.
- Automated Visual Inspection:
Traditionally, QA teams inspect pills, tablets, and capsules manually, which can be time-consuming and prone to human error. ML models, trained using images of both good and defective products, can automate this process. These systems can detect issues like cracks, discoloration, or size inconsistencies much faster and more accurately than humans.
- Anomaly Detection:
Machine learning algorithms can learn what “normal” production data looks like and detect anomalies in real-time, such as slight shifts in chemical compositions or environmental conditions.
These changes might not be noticeable at first but can affect product quality over time.
- Process Optimization:
Machine learning models can continuously learn from data to optimize manufacturing processes. By identifying inefficiencies or points of variability, ML can recommend adjustments to the process, leading to better consistency and quality across different batches.
- Supply Chain QA:
Machine learning can also be used in ensuring the quality of raw materials and managing suppliers. It can predict which suppliers or batches of raw materials are likely to meet quality standards, ensuring the input materials are of effective level.
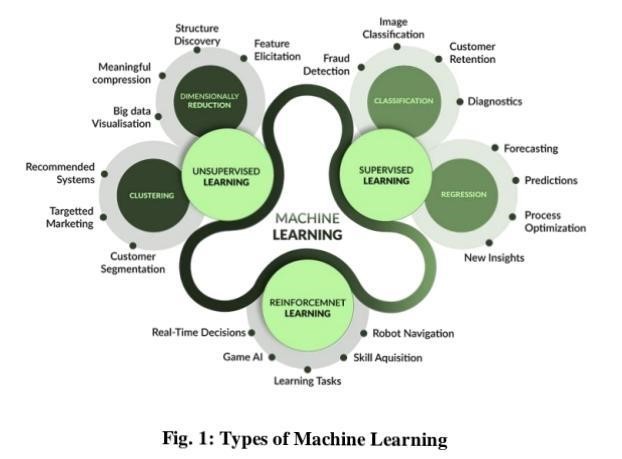
APPLICATION
Artificial intelligence and machine learning:
- Automated Quality Control
Image Recognition and Analysis: AI can analyze images from production lines (e.g., tablet inspections) to detect defects, such as cracks, discoloration, or incorrect sizes. This enhances precision and consistency over manual inspections.
Real-time Monitoring: Machine learning algorithms monitor production equipment and environmental conditions in real time, ensuring optimal operational conditions to prevent product quality deviations.
- Predictive Quality and Risk Management
Predictive Maintenance: AI predicts equipment failure by analysing patterns in equipment data, allowing for proactive maintenance, reducing downtime, and ensuring continuous quality control.
Predictive Product Quality: Machine learning models can forecast product quality issues based on historical production data, allowing early intervention before a product batch fails to meet quality standards.
- Process Optimization and Control
Process Analytical Technology (PAT): AI-driven PAT systems continuously collect data during manufacturing (e.g., temperature, pressure) to adjust processes in real-time, ensuring that the end products meet predefined quality criteria.
Digital Twins: Using AI, digital replicas of the manufacturing process can simulate potential outcomes and optimize settings without interrupting production, helping maintain product consistency and quality.
- Data Integrity and Compliance
Automated Documentation: Natural Language Processing (NLP) automates the review of regulatory documents, ensuring compliance with guidelines from agencies like the FDA or EMA.
Deviation and Anomaly Detection: Machine learning algorithms detect anomalies in production data or deviations in batch records, flagging potential non-compliance issues for early correction. 5. Pharmacovigilance
Adverse Event Detection: AI can process vast amounts of data from clinical trials, patient reports, and post-market surveillance to detect potential adverse effects faster and more accurately than traditional methods.
Signal Detection: Machine learning models help identify trends in adverse drug reaction reports, allowing quicker action on safety issues and reducing risks to patients.
- Intelligent Decision Support Systems (DSS)
Batch Release Decisions: AI-driven decision support systems assist QA teams by evaluating real-time production data and historical performance, guiding decisions on whether to release or hold a batch based on compliance with quality standards.
Risk Assessment: Machine learning models assist in risk-based decision-making by analysing potential quality risks across the product lifecycle, leading to more informed and data-driven QA practices.
- Continuous Quality Improvement
Root Cause Analysis: AI tools help identify root causes of recurring quality issues in manufacturing by analysing complex datasets, leading to process improvements and more consistent product quality.
Continuous Process Verification (CPV): Machine learning ensures that every step in the manufacturing process remains under control, continuously verifying that products are produced according quality specifications.
- Supply Chain Quality Assurance
Supplier Quality Monitoring: AI evaluates and monitors supplier performance, ensuring that raw materials meet required quality standards. Machine learning models can predict which suppliers are more likely to provide materials with quality issues.
End-to-End Traceability: AI enables tracking of products throughout the supply chain, identifying potential risks to product quality, such as improper storage or transportation conditions.
By integrating AI and machine learning into these areas, pharmaceutical companies can improve efficiency, reduce human error, ensure regulatory compliance, and maintain consistent product quality throughout the manufacturing process.
RISK MANAGEMENT
The application of Al in Pharmaceuticals has the potential to speed up treatment identification and optimize clinical trials, benefiting patient outcomes. Despite this, challenges like data privacy, biases, regulatory issues, and ethical concerns about decision-making need addressing. Machine learning (ML) algorithms can evaluate and reduce supply chain risks, including those related to raw material quality and supplier dependability. By examining data from diverse sources, including supplier performance records and quality test results, machine learning models can detect possible hazards and offer suggestions for their mitigation. For instance, ML algorithms might examine past supplier performance data to find trends pointing to dependability problems. Companies should take proactive steps to guarantee the quality of raw materials by identifying high-risk suppliers and looking for alternative sources or stepping up inspections.
FUTURE PROSPECT
The future prospects of AI and machine learning in pharmaceutical quality assurance (QA) are promising, with several exciting developments on the horizon. Below are some key areas where advancements are expected:
- Widespread Adoption of AI-Driven Quality by Design
AI for Process Optimization: AI will become a core component of Quality by Design helping to design pharmaceutical manufacturing processes that are inherently optimized for quality. This includes real-time adjustments to manufacturing variables to ensure consistent products
- Integration of AI with Advanced Analytics
Smart Manufacturing with IoT: The integration ofAI with the Internet of Things will enable continuous quality monitoring by collecting and analysing data from smart sensors embedded in manufacturing equipment. This will allow for seamless tracking of quality parameters across the production lifecycle.
- Regulatory Frameworks for AI in QA
Evolving Regulatory Guidelines: Regulatory bodies such as the FDA and EMA are expected to develop more comprehensive frameworks for the validation, approval, and monitoring of AI-based systems in pharmaceutical QA. This will provide clearer guidance on how AI can be effectively integrated while maintaining compliance with safety and quality
- Enhanced Predictive and Prescriptive Analytics
Advanced Predictive Models: Machine learning models will evolve to provide even more accurate predictions of potential quality issues, allowing for earlier detection of deviations in manufacturing processes. This will reduce product recalls and ensure higher compliance with regulatory standards.
- AI in Real-Time Release Testing (RTRT)
Automated Release Decisions: AI systems will increasingly be used to make real-time decisions on whether a batch of drugs meets quality standards, reducing the need for traditional end-of-line testing. This will speed up product release times and enhance manufacturing agility.
- AI in Personalized and Biopharmaceuticals
Precision QA for Biologics: As the production of biologics and gene therapies expands, AI will be essential in ensuring the complex quality requirements of these products. AI’s ability to analyze large datasets from biological production processes will be critical for maintaining consistency.
CONCLUSION
The integration of AI and machine learning into pharmaceutical quality assurance presents a transformative opportunity to enhance efficiency, accuracy, and compliance within the industry. By leveraging advanced algorithms, predictive models, and automated systems, AI enables more precise monitoring of product quality, reduces human error, and accelerates the decision-making process. These technologies can streamline the traditionally labor-intensive QA processes, ensuring more consistent adherence to regulatory standards while reducing costs. We anticipate a future where QC processes are more resilient, dependable, and capable of guaranteeing the highest standards of product quality and patient safety as the pharmaceutical sector embraces AI and ML. Cooperation amongst technology suppliers, pharmaceutical companies, and regulatory agencies will be crucial to fully realize this potential and guarantee the ethical and efficient application of AI and ML in pharmaceutical.
REFERENCE
- Asifullah Khan, Anabia Sohail, Umme Zahoora, AqsaSaeed Qureshi, “A Survey of the Recent Architectures of Deep Convolutional Neural Networks”, Computer Vision and Pattern Recognition, Available at https://arxiv.org/ftp/arxiv/papers/1901/1901.06032.pdf [Accessed Mar. 13, 2020].
- Ross Girshick, Jeff Donahue, Trevor Darrell, Jitendra Malik, “Rich Feature Hierarchies for ccurate Object Detection and Semantic Segmentation”, IEEE Conference on Computer Vision and Pattern Recognition, pp.580-587,
- Li Liu, Wanli Ouyang, Xiaogang Wang, Paul Fieguth, Jie Chen, Xinwang Liu, Matti Pietikainen, “Deep Learning for Generic Object Detection: A Survey”, International Journal of Computer Vision, vol.128, pp.261-318 2020.
- Kaimin He, Xiangyu Zhang, Shaoqing Ren, Jian Sun, “Spatial Pyramid Pooling in Deep Convolutional Networks for Visual Recognition”, European Conference on Computer Vision, Part 3, pp.346-361,2014.
- Licheng Jiao, Fan Zhang, Fang Liu, Shuyuan Yang, Lingling Li, Zhizi Feng, Rong Qu, “A Survey of Deep Learning-based Object Detection”, IEEE Access, vol.7, pp.128837-128868, , 2019.
- David G. Lowe, “Distinctive Image Features from ScaleInvariant Keypoints”, International Journal of Computer Vision, vol.60, pp.91-110, 2004.
- Juan Du, “Understanding of Object Detection based on CNN Family and YOLO”, Journal of Physics, Conference Series, vol.1004, issue.1, 2018.
- Tsung-Yi Lin, Priya Goyal, Roos Girshick, Kaiming He, Piotr Dollar, “Focal Loss for Dense Object Detection”, International Conference on Computer Vision, pp.2999- 3007, 2017.
- Herbert Bay, Andreas Ess, Tinne Tuytelaars, Luc Van Gool, “Speeded-Up Robust Features (SURF)”, Computer Vision and Image Understanding, vol.110, issue.3, pp.346-359, 2008.
- Alex Krizhevsky, Ilya Sutskever, Geoffrey E. Hinton, “ImageNet Classification with Deep Convolutional Neural Networks”, Communications of the ACM, vol.60, no.6, 2017.
- Yurong Yang, Huajun Gong, Xinhua Wang, Peng Sun, “Aerial Target Tracking Algorithm Based on Faster RCNN Combined with Frame Differencing”, Aerospace, vol.4, no.32, 2017.
- Kwanghyun Kim, Sungjun Hong, Baehoon Choi and Euntai Kim, “Probabilistic Ship Detection and Classification using Deep Learning”, Applied Sciences, vol.8, no.6, 2018.
- Rohith Gandhi, “R-CNN, Fast R-CNN, Faster R-CNN, YOLO - Object Detection Algorithms,” 2018. Available at https://towardsdatascience.com/r-cnn-fast-r-cnnfasterr- cnn-yolo-object-detection-algorithms36d53571365e. [Accessed: Mar. 13, 2020].
- N. Dalal, B. Triggs, “Histograms of Oriented Gradients
- Alexey Bochkovskiy, Chien-Yao Wang, Hong-Yuan Mark Liao, “YOLOv4: Optimal Speed and Accuracy of Object Detection” ,2020.
- Mingxing Tan Ruoming Pang Quoc V. Le Google Research, Brain Team. “EfficientDet: Scalable and Efficient Object Detection”. 2020
- Chien-Yao Wang , Alexey Bochkovskiy, and Hong-Yuan Mark Liao, Institute of Information Science, Academia Sinica, Taiwan, YOLOv7: Trainable bag-of-freebies sets new state-of-the-art for real-time object detectors. 2022.
- https://jonathan-hui.medium.com/object-detection-speedand-accuracy-comparison-faster-r-cnn-r-fcn-ssd-andyolo-5425656ae359.


 Sadiya Inamdar *
Sadiya Inamdar *
 Harshada Gujar
Harshada Gujar


 10.5281/zenodo.14186540
10.5281/zenodo.14186540