Abstract
The emergence of the 2022 multi-country monkeypox outbreak has presented a significant public health challenge, compounding the ongoing coronavirus disease 2019 (COVID-19) pandemic. The disease has rapidly spread across six continents, affecting 104 countries worldwide, with the highest incidence observed in North America and Europe. The etiologic agent, monkeypox virus (MPXV), was first identified in 1959 following isolation from infected monkeys. While human-to-human transmission has been documented since the 1970s, primarily in endemic regions of West and Central Africa, the recent outbreak has exhibited unprecedented rates of transmission, raising concerns about its potential for community spread in non-endemic areas. To mitigate the impact of this re-emerging viral disease, healthcare workers, public health policymakers, and the general public must possess a thorough understanding of its epidemiology, etiology, pathogenesis, clinical manifestations, diagnosis, and management.
Keywords
Emergency, International concern, Prevention, Zoonosis
Introduction
The World Health Organization (WHO) declared a multi-country outbreak of human monkeypox in May 2022, marking a significant public health concern. As of September 21, 2022, a cumulative total of 64,290 laboratory-confirmed cases and 20 deaths had been reported in 106 countries worldwide, underscoring the rapid spread and global impact of this viral disease.[1]
The etiologic agent, monkeypox virus (MPXV), was first identified in 1959 following outbreaks among captive monkeys in Denmark. While human infections were initially documented in Central and Western Africa, the 2022 outbreak has demonstrated unprecedented rates of transmission and community spread in non-endemic regions.[2]
Several factors have contributed to the increased frequency of monkeypox outbreaks in recent decades. The cessation of smallpox vaccination, which conferred significant protection against monkeypox, has rendered populations more susceptible. Additionally, the consumption of animals as a protein source, particularly in regions affected by poverty and social instability, has increased the risk of exposure to MPXV reservoirs. Other contributing factors include population growth, globalization, and ecological changes, such as deforestation, which may facilitate contact with reservoir animals.[3]
Virology and Genomic Classification
Monkeypox virus (MPXV) is classified within the genus Orthopoxvirus, family Poxviridae, alongside variola, cowpox, and vaccinia viruses. A distinctive characteristic of MPXV compared to other orthopoxviruses is its broad host range, encompassing diverse species from rope squirrels to sooty mangabeys. This extensive host tropism may have facilitated prolonged zoonotic circulation of MPXV in the wild.[4] The name "monkeypox" originated from the Initial isolation in 1958 from infected cynomolgus monkeys [5]. However, recent evidence suggests this nomenclature might be misleading. Serological data from animal samples points towards rodents as the primary natural reservoirs, with primate infections representing spillover events [6] Additionally, the ongoing global outbreak highlights the potential for direct human-to-human transmission. Phylogenetic analyses of recent isolates reveal genomic divergence from the original monkey-infecting MPXV strains. These factors are prompting discussions regarding a potential name change for MPXV [7].
Despite this, inoculation of MPXV into non-human primates remains a valuable animal infection model for poxviruses. It induces symptoms closely resembling human smallpox, albeit milder and with reduced transmission rates. Research into smaller animal models, such as BALB/c mice, is also ongoing [8].
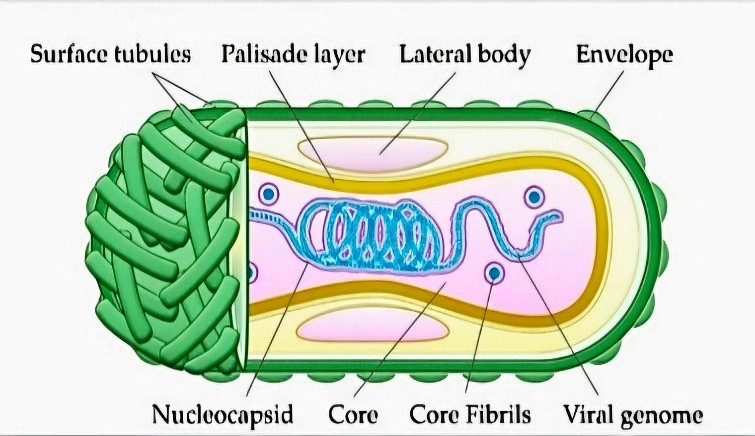
Fig 1 - MPVX Virion Structure
Electron microscopy reveals MPXV to possess the characteristic ovoid or rectangular brick-shaped morphology of poxviruses, measuring
approximately 200 x 250 nm. The virion surface exhibits membrane tubules or filaments, and the core component appears biconcave [9].
The MPXV genome consists of a linear, double-stranded DNA molecule with a substantial length exceeding 197 kb. This large size, containing around 200 genes, presents a significant challenge for de novo whole-genome assembly [10]. The viral genome encodes all proteins necessary for replication and structural assembly. It is covalently closed at both ends by inverted terminal repeats (ITRs) of roughly 10 kb each. As observed in other orthopoxviruses, sequence conservation is high in the central region of the MPXV genome but diminishes towards the terminal ITRs. The central region harbors genes responsible for housekeeping functions and thus exhibits high conservation among orthopoxviruses. Conversely, genes encoding proteins that interact with host factors demonstrate lower sequence identity and are located closer to the termini [11]. These latter coding regions are aptly named virulence factors, as most appear dispensable for in vitro replication in cell culture, but their absence attenuates pathogenesis in vivo [12]. Based on sequence homology, MPXV isolates from the African continent can be categorized into two clades: West African and Congo Basin (or Central African) strains. Inter-clade sequence homology is approximately 95%, while intra-clade homology approaches 99% [13].These clades exhibit variations in clinical presentation, disease severity, and transmission patterns, in addition to their geographical distribution. The West African clade was previously considered milder, with no reported mortalities until the 2017-2018 Nigerian outbreak. In contrast, the Congo Basin clade historically displayed a case-fatality ratio of around 10% [9, 34]. Initial phylogenetic investigations suggest that the current 2022 outbreak is primarily associated with the West African clade [14]. To avoid geographically discriminatory identification, a revised nomenclature system for MPXV clades has been proposed. suggest designating isolates from the Congo Basin as MPXV Clade 1 and those

Fig 2 - Strong Evidence of human-to-human transmission of MPVX
originating from West Africa as Clades 2 and 3. This three-clade classification system is adopted here . Notably, virulence differs between the clades, with Clades 2 and 3 exhibiting lower virulence and transmission rates in humans and non-human primates (NHPs) compared to Clade 1. These observations may explain the absence of fatalities in the 2003 US outbreak [15]. Additionally, despite similar non-vaccinated seroprevalence between both regions, roughly 90% of reported cases historically originated from the Congo Basin. However, the ongoing 2022 outbreak demonstrates signs of divergence from the original two clades, particularly regarding human-to-human transmission efficiency. This subclade branched from Clade 2 and is currently designated as Clade 3 or “human MPXV” (hMPXV).
The most significant diversity between Clade 1 and Clade 2 (and 3) appears concentrated in the terminal regions flanking the ITRs, which contain genes encoding host-response modifier (HRM) proteins. One such protein is the monkeypox ortholog of the poxviral inhibitors of complement enzymes (PICEs)
Pathophysiology and Immune Evasion
The Monkeypox Virus (MPXV) employs multiple entry routes into the human body, including the oropharynx, nasopharynx, and dermis. Notably, sexual transmission has also been identified as a potential mode of infection. Human-to-human transmission can occur through direct contact with infected skin lesions or mucosa, respiratory droplets, or indirect contact with contaminated materials.[16]
Following entry, the virus replicates at the inoculation site and spreads to regional lymph nodes. After an incubation period of 1-3 weeks, symptoms such as backache, sore throat, shortness of breath, fever, chills, malaise, headache, and enlarged lymph nodes may appear. Approximately 1-3 days after the onset of fever and lymphadenopathy, the infectious stage begins with the development of a rash, often starting on the face and spreading to other body parts.[17]
Like other orthopoxviruses, MPXV has evolved various mechanisms to evade the host’s immune defenses, facilitating its entry and replication without detection. One such mechanism involves the disruption of pattern recognition receptors (PRRs) expressed by innate immune cells. PRRs, including Toll-like receptors (TLRs), NOD-like receptors (NLRs), RIG-1-like receptors (RLRs), and C-type lectin receptors (CLRs), are crucial for recognizing microbial-associated molecules and initiating inflammatory responses.[18]
Orthopoxviruses can produce proteins that interfere with the signaling pathways of PRRs. For instance, MPXV produces the A47R protein, which can interact with MyD88, TRIF, and TRAM, key adaptor proteins involved in PRR signaling. This interaction disrupts the normal function of these adaptor proteins, leading to the inhibition of transcription factors such as NF-?B and ultimately hindering the activation of the innate immune response.
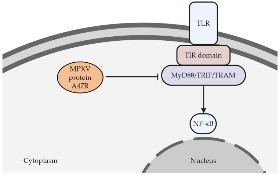
Fig 3 – Pathophysiology of Monkeypox
Orthopoxviruses, including monkeypox virus (MPXV), have evolved strategies to evade the host’s immune response. One such tactic involves inhibiting apoptosis, a cellular process that eliminates infected cells to prevent viral proliferation. The viral proteins produced by orthopoxviruses can disrupt various mechanisms involved in apoptosis.[17
NF-?B, a crucial transcription factor, plays a pivotal role in regulating both inflammation and apoptosis. MPXV’s ability to inhibit NF-?B activity not only impairs the immune response but also interferes with apoptosis pathways. Additionally, orthopoxviruses can target specific caspases, enzymes essential for executing apoptosis. Viral proteins such as B12R and C7L in MPXV strain Zaire-I-96 can inhibit the activity of caspases 1, 8, and 9. Furthermore, orthopoxviruses can produce proteins that mimic the function of Bcl-2 proteins, a family of proteins known for their anti-apoptotic properties. MPXV’s P1L protein interacts with the I?B kinase (IKK) complex, a key regulator of NF-?B activation, thereby hindering apoptosis. Moreover, orthopoxviruses can interfere with the production of interferon, a crucial antiviral molecule. By blocking interferon regulatory factors (IFRs), these viruses can suppress the host’s innate immune response. In summary, orthopoxviruses employ multiple strategies to evade the host’s immune response, including inhibiting apoptosis and interfering with inflammatory pathways. These mechanisms contribute to the virus’s ability to establish infection and spread.[18]
DIAGNOSIS
The diagnosis of monkeypox requires a comprehensive evaluation, including a detailed medical history and a thorough clinical assessment. A travel history to endemic regions, contact with infected animals or individuals, and the presence of nonspecific symptoms such as fever, headache, myalgia, asthenia, and a rash should be carefully considered.[19]
Given the wide range of differential diagnoses for acute rash accompanied by nonspecific symptoms, it is crucial to consider conditions such as varicella, measles, molluscum contagiosum, cutaneous bacterial infections, scabies, syphilis, drug allergies, and sexually transmitted infections (STIs). Lymphadenopathy during the prodromal phase can help differentiate monkeypox from varicella and variola. Real-time polymerase chain reaction (PCR) is the preferred laboratory method for detecting MPXV. PCR testing is highly sensitive and can be performed on specimens obtained from lesion exudate or scabs. While other methods like virus isolation, immunohistochemistry, ELISA, and electron microscopy can be used, they often require specialized facilities and expertise.[20]
Treatment
Monkeypox infections are often self-limiting, and supportive care is generally sufficient. Individuals with mild symptoms and no risk factors for severe disease can be managed at home, provided that infection prevention and control measures are followed. For mild or uncomplicated cases, symptomatic relief can be provided using antipyretics, analgesics, or antiemetic medications. Adequate hydration, nutritional assessment, and vaccination review are essential, particularly in pediatric patients. Vitamin A supplementation may be beneficial for individuals with deficiencies, as it plays a crucial role in wound healing.[21] Mild skin rashes can be treated supportively to alleviate irritation and promote healing. Antimicrobial agents should be considered if a secondary bacterial infection is suspected. Complications such as cellulitis, necrotizing soft tissue infection, or abscess require appropriate monitoring and treatment.
Mental health support is essential for patients with monkeypox, as prolonged isolation can lead to anxiety and depression.
High-risk patients, including children, pregnant women, immunocompromised individuals, and those with poor skin integrity, should be hospitalized for monitoring and considered for antiviral treatment. Confluent rashes or skin lesions exceeding a hundred, as observed in smallpox studies, may indicate severe disease.[22]
Patients with progressive illness or complications, such as severe dehydration, pneumonia, encephalitis, ocular lesions, or sepsis, require antiviral agents and specific treatment.
Prevention
Monkeypox is primarily transmitted through direct contact with infected individuals or animals, undercooked meat, or contaminated objects. Respiratory droplets, skin or mucosal lesions, blood, and bodily fluids can serve as sources of infection. Recent evidence suggests that monkeypox can also be sexually transmitted, particularly among men who have sex with men. A study in Italy found viral DNA in the semen of infected patients for at least nine days after symptom onset, although infectivity remains uncertain. Mother-to-child transmission has also been reported.To prevent the spread of monkeypox, individuals should practice hand hygiene, avoid sharing personal items, and maintain a distance of at least one meter from suspected or confirmed cases. Caregivers should wear appropriate personal protective equipment, including masks and gloves. Infected patients should remain in isolation until all skin lesions have crusted and scabs have fallen off. However, the virus may persist in bodily fluids, so quarantine may need to be extended for up to six weeks. Sexually active patients are advised to use condoms for 12 weeks after recovery.[21] Patients with monkeypox should be isolated in a well-ventilated space separate from others. Caregivers should be in good health, vaccinated against smallpox, and provided with guidance on disease transmission and self-prevention. Poxviruses can persist on household items, especially in dark, cool, and dry environments. Disinfectants should be used to clean all areas where the infected patient has been. Porous surfaces may retain live viruses longer than nonporous surfaces. The patient’s clothing and bedding should be washed with soap and hot water. Avoid shaking, dry dusting, sweeping, or vacuuming to prevent the aerosolization of virus particles. The patient’s waste should be placed in a secured bag and treated with chlorine to reduce contamination.[23]
Vaccines and Vaccination
The eradication of smallpox, a significant achievement in modern medicine, was made possible through effective vaccination programs. Following the eradication in 1980, routine vaccination of the general population was discontinued after careful consideration of risks and benefits. As a result, a large portion of the global population lacks protection against orthopoxviruses, including the monkeypox virus. Given the increasing number of MPXV infections worldwide, the Advisory Committee on Immunization Practices (ACIP) recommended pre-exposure prophylaxis for healthcare workers, laboratory personnel, and others at risk of contracting the virus. This review focuses on the efficacy and safety of the ACIP-recommended vaccines, ACAM2000 and JYNNEOS.[24] The U.S. Strategic National Stockpile (SNS) currently holds over 100 million doses of ACAM2000 and more than 1000 doses of JYNNEOS. Globally, the Smallpox Vaccine Emergency Stockpile (EVES) consists of approximately 2.4 million doses held by the WHO and over 30 million doses pledged by various donor countries.[24]
Acknowledgement
Authors take opportunity to express their sincere gratitude and deep sense of indebtedness towards Associate professor Hon Ms. Bhagyashri Randhwan &Director cum Principal Hon. Mr. Yogesh Bafanasir, at Arihant College of Pharmacy Kedgaon, Ahmednagar who has always inspired us and extended theirfull Co – operation in research work, Further.
REFERENCE
- WHO | World Health Organization WHO Director-General’s Statement at the Press Conference Following IHR Emergency Committee Regarding the Multi-Country Outbreak of Monkeypox, 23 July 2022.
- Hammarlund E., Lewis M.W., Carter S.V., Amanna I., Hansen S.G., Strelow L.I., Wong S.W., Yoshihara P., Hanifin J.M., Slifka M.K. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox.
- Rimoin Anne W., Mulembakani Prime M., Johnston Sara C., Lloyd Smith James O., Kisalu Neville K., Kinkela Timothee L., Blumberg S., Thomassen Henri A., Pike Brian L., Fair Joseph N., et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo.
- Falendysz E.A., Lopera J.G., Doty J.B., Nakazawa Y., Crill C., Lorenzsonn F., Kalemba L.s.N., Ronderos M.D., Mejia A., Malekani J.M., et al. Characterization of Monkeypox virus infection in African rope squirrels (Funisciurus sp.) PLoS Negl.
- Magnus P.v., Andersen E.K., Petersen K.B., Birch-Andersen A. A pox-like disease in cynomolgus monkeys. Pathol. Microbiol. Scand.
- Reynolds M.G., Doty J.B., McCollum A.M., Olson V.A., Nakazawa Y. Monkeypox re-emergence in Africa: A call to expand the concept and practice of One Health. Expert Rev. Anti. Infect. Ther.
- Happi C., Adetifa I., Mbala P., Njouom R., Nakoune E., Happi A., Ndodo N., Ayansola O., Mboowa G., Bedford T., et al. Urgent Need for a Non-Discriminatory and Non-Stigmatizing Nomenclature for Monkeypox Virus.
- Osorio J.E., Iams K.P., Meteyer C.U., Rocke T.E. Comparison of monkeypox viruses pathogenesis in mice by in vivo imaging.
- Sklenovská N. Monkeypox Virus. In: Malik Y.S., Singh R.K., Dhama K., editors. Animal-Origin Viral Zoonoses. Springer; Singapore.
- Zhao K., Wohlhueter R.M., Li Y. Finishing monkeypox genomes from short reads: Assembly analysis and a neural network method.
- 11]]Remichkova M. Poxviruses: Smallpox vaccine, its complications and chemotherapy. Virus Adaptation Treat.
- 12]Haller S.L., Peng C., McFadden G., Rothenburg S. Poxviruses and the evolution of host range and virulence.
- 13]Likos A.M., Sammons S.A., Olson V.A., Frace A.M., Li Y., Olsen-Rasmussen M., Davidson W., Galloway R., Khristova M.L., Reynolds M.G., et al. A tale of two clades: Monkeypox viruses.
- Giorgi F.M., Pozzobon D., Di Meglio A., Mercatelli D. Genomic characterization of the recent monkeypox outbreak.
- Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., Wegner M.V., Kazmierczak J.J., Stratman E.J., Li Y., Fairley J.A., et al. The Detection of Monkeypox in Humans in the Western Hemisphere.
- McCollum A.M., Damon I.K. Human monkeypox.
- Di Giulio D.B., Eckburg P.B. Human monkeypox.
- Shchelkunov S.N. Orthopoxvirus genes that mediate disease virulence and host tropism.
- Di Gennaro F., Veronese N., Marotta C., Shin J.I., Koyanagi A., Silenzi A., Antunes M., Saracino A., Bavaro D.F., Soysal P., et al. Human Monkeypox: A Comprehensive Narrative Review and Analysis of the Public Health Implications. Microorganisms.
- Petersen E., Kantele A., Koopmans M., Asogun D., Yinka-Ogunleye A., Ihekweazu C., Zumla A. Human monkeypox: Epidemiologic and clinical characteristics, diagnosis, and prevention.
- Polcz M.E., Barbul A. The Role of Vitamin A in Wound Healing.
- Penner F., Henderson D.A., Arita I., Jezek Z., Ladnyi I.D., World Health O. Smallpox and Its Eradication.
- World Health Organization Clinical Management and Infection Prevention and Control for Monkeypox.
- Rao A.K., Petersen B.W., Whitehill F., Razeq J.H., Isaacs S.N., Merchlinsky M.J., Campos-Outcalt D., Morgan R.L., Damon I., Sánchez P.J., et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses.


 Tejas Bagmar*
Tejas Bagmar*



 10.5281/zenodo.14173467
10.5281/zenodo.14173467