Abstract
Nanotechnology, the manipulation of matter on an atomic and molecular scale, has emerged as a revolutionary force in various fields, including medicine. In particular, its application in cancer diagnosis and treatment has shown immense potential to address the limitations of conventional therapies. Historically, chemotherapy has been a mainstay in cancer treatment. However, it often suffers from systemic toxicity, drug resistance, and poor drug delivery. Nanotechnology offers innovative solutions to these challenges by enabling the development of targeted drug delivery systems. Nanoparticles, with their unique properties, can be designed to carry therapeutic agents directly to cancer cells, minimizing damage to healthy tissues. This review delves into the significant role of nanotechnology in contemporary cancer management. By analyzing a comprehensive range of research articles published between 2002 and 2021, this paper highlights the breakthroughs, limitations, and prospects of nanoparticle-based therapies. The inclusion criteria for the selected articles were based on their relevance to nanoparticle applications in cancer treatment, ensuring a focused and informative analysis.
Keywords
Cancer therapy, Cancer treatment, Multidrug resistance, Nanoparticles, Nanotechnology, Nanocarriers, Nanomedicine.
Introduction
Cancer is a group of diseases characterized by the uncontrolled growth and spread of abnormal cells. These cells can invade nearby tissues and spread to other parts of the body. [1] Cancer is a group of diseases characterized by the uncontrolled growth and spread of abnormal cells. Over the years, extensive research has been conducted to identify various factors that contribute to the development of cancer. While certain cancers have been linked to specific environmental factors such as radiation and pollution, lifestyle factors such as a poor diet, tobacco use, smoking, stress, and lack of physical activity play a significant role in determining cancer risk. [2] According to a 2018 report by the World Health Organization (WHO), cancer is the second leading cause of death globally, with over 18 million new cases and nearly 10 million cancer-related deaths. With the rapid pace of industrialization, cancer mortality rates are projected to nearly double by 2040. [3] Cancer is a major global health concern and the second leading cause of death worldwide. According to the American Cancer Society, it is estimated that 1.9 million new cases of cancer will be diagnosed in 2021. [4] Conventional cancer treatments include surgery, chemotherapy, radiation therapy, targeted therapy, immunotherapy, and hormone therapy. [5]
Novel Tumor Targeting Nanoparticles:
Tumors are complex tissues composed of cancer cells and, in the case of carcinomas, a supportive stroma containing various cell types such as fibroblasts, myofibroblasts, endothelial cells, pericytes, and immune cells.[6]
Types of Nanoparticles as Drug Delivery Systems.
Nanoparticles can be made from various materials, including polymers, metals, and ceramics. Their properties can vary based on their manufacturing methods and materials. Many types of nanoparticles, such as liposomes, lipid-based carriers (like lipid emulsions and lipid-drug complexes), polymer-drug conjugates, polymer microspheres, micelles, and ligand-targeted products, are being developed as drug delivery systems.[7]
Novel tumor targeting methods:
1. Nanotechnology.
2.Chimeric antigen receptor(CAR) T CELL Therapy.
3. Antibody drug conjugates. (ADCS).
4. Tumor microenvironment targeting.
5. Oncolytic viruses
6. RNA-based therapies
7. CRISPR and gene editing.
Multifunctional Nanoparticles for Tumor Imaging
Tumor imaging is crucial in clinical oncology, as radiological examinations help detect solid tumors, monitor their recurrence, and assess treatment responses. Conventional imaging techniques like CT and MRI primarily focus on the morphological features of tumors, such as their location, size, and extent. While advancements in imaging technology have improved spatial resolution, these techniques often lack the sensitivity and specificity to provide detailed functional information about the disease. This limitation hinders early diagnosis and effective monitoring of treatment responses. [8]
Nanoparticles (NPs) are particles with at least one dimension less than 100 nanometers. These tiny particles exhibit unique properties that are not typically found in larger-scale materials. Based on their overall shape, nanoparticles can be classified as 0D, 1D, 2D, or 3D.[9]
Nanoparticles are submicrometer-sized colloidal systems, often made of polymers (both biodegradable and non-biodegradable). Depending on the preparation method, nanoparticles can be classified as nanospheres or nanocapsules. While nanospheres are matrix systems where the drug is dispersed throughout the particle, nanocapsules are vesicular systems with an aqueous or oily core surrounded by a polymeric membrane. This makes nanocapsules a ‘reservoir’ system for drug delivery.[10]
Nanoparticles typically consist of a core, a shell layer, and a surface layer. The core is the central part of the nanoparticle and is often referred to as the nanoparticle itself. Nanoparticles have shown promise in deep tissue penetration, enhancing the EPR (Enhanced Permeability and Retention) effect. [11]
Synthesis of NPs
Nanoparticles come in various shapes, sizes, and structures. Numerous synthesis methods are used to achieve these variations, which can be broadly categorized into two major groups.
1) bottom-up approach and
2) top-down approach.
These approaches can be further classified into different subclasses based on reaction conditions and operation.
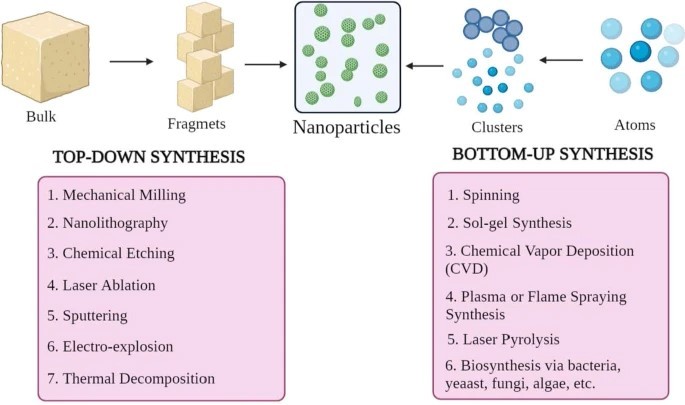
• Top-Down Approach
This method, also known as the top-down approach, involves breaking down larger materials into smaller nanoparticles. Techniques such as mechanical milling, nanolithography, chemical etching, laser ablation, sputtering, electro-explosion, and thermal decomposition are used to achieve this.
•Bottom?up Approach
This method, also known as the bottom-up approach, involves building nanoparticles from smaller units, such as atoms or molecules. Common techniques include spinning, sol-gel synthesis, chemical vapor deposition (CVD), plasma or flame spray pyrolysis, laser pyrolysis, and biosynthesis.
ORGANIC NPs:
Organic nanoparticles, made from various materials, have been extensively researched for decades. Some of the most studied organic nanoparticles include liposomes, polymer nanoparticles, and dendrimers.[12]
1. LIPOSOMES:
Liposomes are the first nanoscale drug that was approved in 1965.
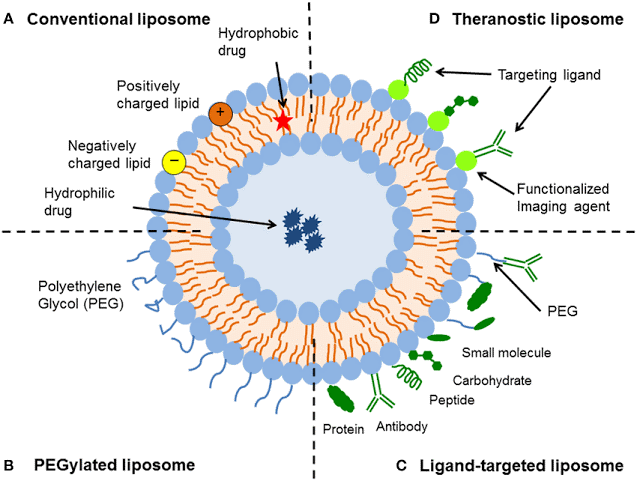
Liposomes are one of the most extensively researched drug delivery systems. Their unique structure, consisting of an aqueous core surrounded by a phospholipid bilayer, enables them to deliver both water-soluble and fat-soluble drugs.
Liposomes offer several advantages, including biocompatibility, efficient drug encapsulation, controllable size, and ease of modification. However, their short circulation time is a limitation, which can be addressed through PEGylation. Surface modification also allows for the development of multifunctional liposome-based nanoparticles with improved targeting capabilities for tumors. [13]
2. POLYMERIC BASED NANOPARTICLES :
Polymeric nanoparticles (PNPs) are submicron-sized colloidal particles. Anticancer drugs can be adsorbed, encapsulated, or conjugated within or onto the surface of these nanoparticles. Targeted drug delivery systems utilize drug-loaded PNPs to release therapeutic agents in a sustained manner at specific target sites. The polymer shell protects the drug from degradation by enzymes in the body. [14]
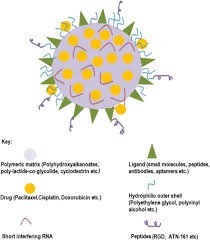
Polymeric micelles are another type of nanoparticle composed of a hydrophobic core and a hydrophilic shell. These micelles are formed through the self-assembly of amphiphilic block copolymers in water. The hydrophobic core can solubilize poorly water-soluble drugs, increasing their bioavailability. The hydrophilic shell prolongs blood circulation time and enhances blood stability. By adding tumor-specific ligands, polymeric micelles can be targeted to tumors, improving drug delivery to cancer cells.
One example of a polymeric micelle is PLGA-PEG-retinoic acid (RA), which has been developed to deliver irinotecan to HT-29 human colorectal and HepG2 cells. [15]
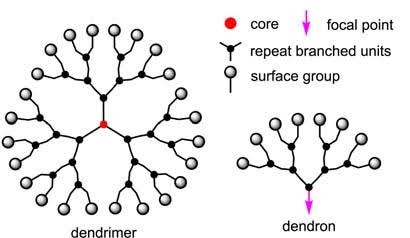
3. DENDRIMERS
Dendrimers are unique, highly branched polymers with a well-defined, three-dimensional structure. They can be synthesized using two primary methods: divergent and convergent synthesis. In divergent synthesis, branches grow radially outward from a central core. A notable example is polyamidoamine (PAMAM) dendrimers, which contain tertiary amine and amide groups that can bind various molecules. In convergent synthesis, dendritic branches are synthesized separately and then attached to a core. Polypropylenimine (PPI) and poly aryl ether dendrimers are commonly synthesized using this method. [16] The synthesis of dendrimers begins with reacting an ammonia core with acrylic acid. This reaction forms a “tri-acid” molecule, which then reacts with ethylenediamine to produce “tri-amine,” the first-generation product. This process is repeated, with the “tri-amine” reacting with acrylic acid to form “hexa-acid,” which in turn yields “hexa-amine” (Generation 1). This iterative process continues, building layer upon layer, to create higher generations of dendrimers. Typically, dendrimers range in size from 1 to 10 nanometers, although some can reach up to 15 nanometers. [17]
HYBRID NANOPARTICLES
Lipid–polymer hybrid nanoparticles:
Lipid-based polymeric nanoparticles (LPHNPs) are highly popular drug delivery systems for cancer therapy. They consist of three main components:
* Polymer core: This central core encapsulates the drug.
* Lipid monolayer: Surrounding the polymer core, this layer reduces drug release and protects the core from water.
* Lipid-PEG layer: This outer layer allows for the conjugation of targeting moieties and prolongs circulation time by evading immune responses.
LPHNPs offer several advantages:
* Structural integrity and stability: The polymeric core provides robust structure and stability during storage.
* Controlled release: The lipid monolayer enables controlled drug release.
* Biocompatibility and bioavailability: The lipid and lipid-PEG layers enhance biocompatibility and bioavailability.
Overall, LPHNPs are effective, convenient, and reliable drug delivery carriers. [18]

TYPES OF LPHNP:
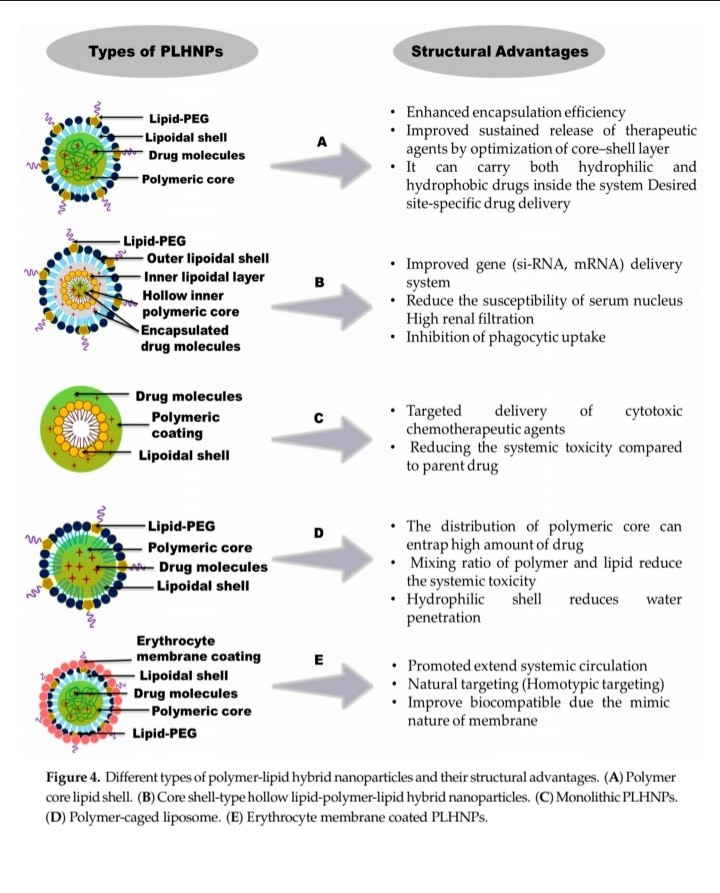
Synthesis methods for LPHNPs Various methods have been developed for the synthesis of LPHNPs, but they can be broadly grouped into two main strategies: (1) Two-step methods and (2) One-step methods [19]
1. Two-step methods
Initially, LPHNPs were synthesized using a two-step method. In this approach, polymeric nanoparticles were mixed with pre-formed liposomes. Electrostatic interactions facilitated the incorporation of the lipid shell onto the surface of the polymer core.
The polymeric core could be prepared through various methods, including nanoprecipitation, emulsification-solvent evaporation, or high-pressure homogenization.
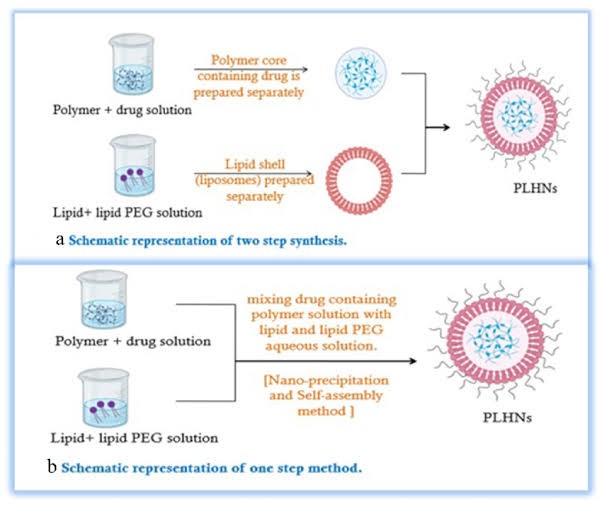
One-step methods
One-step methods, such as nanoprecipitation or emulsification-solvent evaporation, can be used to synthesize LPHNPs. However, nanoprecipitation offers several advantages over emulsification-solvent evaporation, including simplicity and the ability to produce nanoparticles with a narrow size distribution. [20]
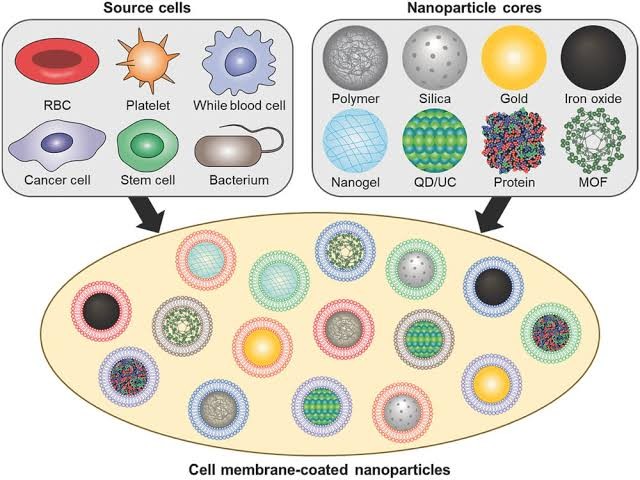
CELL MEMBRANE COATED NANOPARTICLES:
Cell membrane:
CMCNs have been used in cancer immunotherapy. For instance, they can be applied to deliver immunotherapy drugs and immunomodulators to tumors, or directly enhance the efficacy of cancer immunotherapy by their characteristics.
Preparation of CMCNs:
The preparation of CMCNs mainly includes three steps:
1. Separation and preparation of cell membrane-derived vesicles (CMVs),
2. synthesis of nanoparticle cores, and
3. fusion of CMVs and nanoparticle cores. [21]
1. RED BLOOD CELL (RBC)
Red blood cells (RBCs) are the most abundant cells in the blood. They contain hemoglobin, a protein essential for transporting oxygen throughout the body. With a lifespan of approximately 120 days, RBCs are ideal carriers for long-circulating drugs. They were among the first cells used to develop nano-drug delivery systems. [22]
RBC-platelet hybrid membranes coated with polypyrrole demonstrated significantly longer circulation times and improved tumor-targeting ability in vivo.
RBC membranes can also target bacteria and absorb pore-forming toxins (PFTs) released during gram-positive bacterial infections.[23]
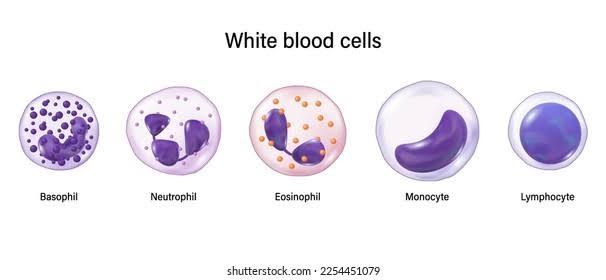
2. WHITE BLOOD CELLS (WBC)
White blood cells, or leukocytes, are larger than red blood cells, measuring between 7 and 20 micrometers in diameter.
White Blood Cell Membrane-Coated Nanoparticles: Recent Development and Medical Applications.
White blood cells (WBCs) are routinely measured in blood tests and are divided into five types: basophils, eosinophils, lymphocytes, monocytes, and neutrophils.[24]
3. PLATELATES
Platelets originate from the fragmentation of mature megakaryocytes. Their plasma membrane contains multifunctional proteins that are essential for their role in blood clotting. The CD47 receptor on platelet membranes is believed to help PMNPs evade detection by macrophages.[25]
Approximately 150,000 to 350,000 platelets are circulating in each microliter of blood, helping to maintain vascular integrity. Platelets have an average lifespan of 8 to 9 days. They play a crucial role in various physiological processes, including hemostasis (blood clotting), wound healing, inflammation, and thrombosis [26]
4. CANCER CELL MEMBRANE COATED NANOPARTICLES:
Cancer cell membranes are ideal candidates for wrapping nanoparticles for cancer therapy. Cancer cells are readily cultured in large quantities in vitro, making it easy to harvest their membranes. Additionally, they possess the unique ability to target other cancer cells, a phenomenon known as homotypic targeting.[27]
5. CANCER STEAM CELL:
Cancer stem cells (CSCs) are highly resistant to conventional cancer treatments like chemotherapy and radiation therapy.
Cancer stem cells (CSCs) are a small population of cells within tumors that possess the ability to self-renew and differentiate into various cell types, ultimately leading to tumor growth, metastasis, and recurrence. These cells are often resistant to conventional cancer therapies.
Cancer stem cell biology :
Cancer is defined as a biological condition in which some cells in a tissue of a bodily organ undergo uncontrolled division and growth. In 1997, Bonnet and Dick realized that a small subpopulation of these abnormal cells has different properties from those of bulk tumor cells. After isolation, they demonstrated that this small population of leukemia-initiating cells has features similar to stem cells and announced the concept of cancer stem cells (CSCs).[28]
Cancer stem cells and their markers:
Targeting cancer stem cells (CSCs) is a promising area of cancer research. Evidence suggests that these cells play a crucial role in tumor growth and progression.[29]
INORGANIC NANOPARTICLES:
Inorganic nanoparticles, with their uniform size distribution and dimensions as small as a few nanometers, are highly promising as passive or active carriers for tumor targeting.[30]
A) GOLD NANOPARTICLES
Gold nanoparticles are highly versatile due to their simple surface chemistry, enabling various modifications. This makes them suitable for the development of biocompatible and functional nanomaterials for cancer therapy.[31]
As the utility of AuNPs largely depends on the degree of inherent toxicity, studies on the toxicological profile of these NPs are discussed proceeding to their usage in cancer management.[32]
B) CARBON NANOTUBES NANOPARTICLES:
Carbon nanotubes, discovered by Iijima in 1991, are cylindrical structures composed of carbon atoms arranged in a hexagonal lattice. These unique structures belong to the fullerene family, the third known allotrope of carbon, alongside graphite and diamond.[33]
Carbon nanotubes are large, cylindrical molecules composed of carbon atoms arranged in a hexagonal lattice. The walls of these nanotubes can consist of single or multiple layers of graphene sheets. Single-
walled carbon nanotubes (SWCNTs) are formed from a single layer of graphene, while multi-walled carbon nanotubes (MWCNTs) consist of multiple layers [34]
CNTs are cylindrical structures composed of one or more concentric layers of graphene sheets. Their diameter typically ranges from 0.4 to 100 nanometers, while their length can reach several micrometers.
CNTs are categorized into two main types:
* Single-walled carbon nanotubes (SWCNTs): Composed of a single layer of graphene.
* Multi-walled carbon nanotubes (MWCNTs): Composed of multiple layers of graphene. [35]

[36]
C)QUANTUM DOTS:
Quantum dots (QDs) are nanometer-sized, luminescent semiconductor nanocrystals. Their unique optical properties, including high brightness, long-term stability, ability to detect multiple signals simultaneously, and tunable emission spectra, make them promising candidates for diagnostic and therapeutic applications in oncology.[37]
Quantum dots (QDs) can serve as nanocarriers for drugs, enabling targeted drug delivery and improving drug bioavailability. QD-based drug delivery systems hold the potential for early detection, monitoring, and localized treatment of specific diseases.[38]
Quantum dots (QDs) are nanoscale semiconductors with a wide bandgap. They are typically composed of metals, lipids, or polymers. While metal QDs have shown promise in early-stage tumor imaging and therapy, concerns about their biological toxicity have led to the development of non-functionalized QDs, including carbon QDs (CQDs), graphene QDs (GQDs), black phosphorus QDs (BPQDs), and perovskite quantum dots (PQDs).
Physical Properties of QDs
Quantum dots (QDs) are semiconductor materials composed of binary and ternary alloys from groups II-VI, III-V, and IV-VI of the periodic table. These nanocrystals, typically ranging from 2 to 20 nanometers in size, offer several advantages:
* Excellent optical stability and long fluorescence lifetimes: QDs maintain their optical properties over time.
* Simultaneous excitation: Multiple QDs can be excited by a single light source.
* Narrow and tunable emission spectra: QDs emit light in narrow, tunable wavelengths with broader absorption spectrum [39]
Conventional quantum dots are typically composed of a metallic core surrounded by a protective coating. The core material, often cadmium selenide (CdSe) or cadmium sulfide (CdS), is responsible for the quantum dot’s fluorescence. While these quantum dots have been extensively studied, they face challenges such as low stability, limited efficacy, and toxicity, primarily due to the use of cadmium.[40]
Carbon dots possess several desirable properties, including high water solubility, strong chemical inertness, ease of processing, and excellent resistance to photobleaching. The term “carbon dots” was first introduced in 2006 by Sun et al., who synthesized fluorescent carbon nanoparticles through laser ablation of carbon targets followed by surface passivation.[41]
The photoluminescence of graphene quantum dots (GQDs) is primarily influenced by edge states, quantum confinement, and surface states. Unlike two-dimensional graphene, which is a semimetal, GQDs exhibit a non-zero bandgap, behaving as semiconductors or insulators. This bandgap opening enhances light absorption and increases the energy spectrum of GQDs.[42]
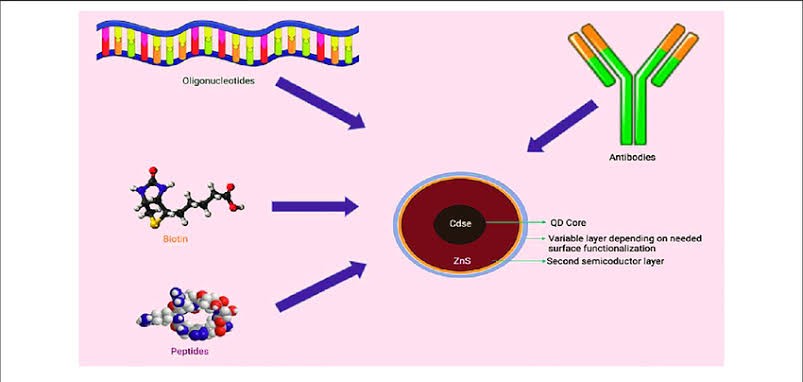
Fig. Schematic Representation of Simple Quant dot Assembly
D) Silica nanoparticles
MESOPOROUS SILICA NANOPARTICLES
Mesoporous silica nanoparticles (MSNs) are promising candidates for cancer diagnosis and therapy due to their large surface area, high pore volume, tunable pore size, abundant surface chemistry, and acceptable biocompatibility. MSN-based delivery systems can improve therapeutic efficacy while reducing toxicity to normal tissues. The unique properties of MSNs make them suitable for delivering both soluble and insoluble anticancer drugs.[43] Monodispersed silica particles are synthesized using a sol-gel method, first reported by Stöber et al. in 1968. This method involves the hydrolysis of tetraalkyl silicates in an alcohol-water solution catalyzed by ammonia. The resulting non-porous silica particles can be engineered to have sizes ranging from a few nanometers to several micrometers.[44]

REFERENCE
- Kaur C., Garg U. Artificial intelligence techniques for cancer detection in medical image processing: A review. Mater. Today Proc. 2021.
- Nanoparticles for Cancer Therapy: Current Progress and Challenges Shreelaxmi Gavas, Sameer Quazi and Tomasz M. Karpi?ski.
- World Health Organization. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; WHO: Geneva, Switzerland, 2020.
- Jing H et al. Formation and properties of self-assembled nanoparticle-supported lipid bilayer probed through molecular dynamics simulations Langmuir, 2020, 36, 5524–33.
- Park W, Heo YJ, Han DK, New opportunities for nanoparticles in cancer immunotherapy. Biomater, 2018, Res 22:24.
- Stromal Fibroblasts in Cancer: A Novel Tumor-Promoting Cell Type Akira Orimo &Robert A. Weinberg Pages 1597-1601.
- Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer 2002; 2: 750.
- Application of Nanotechnology in Cancer Therapy and Imaging Dr. Xu Wang PhD, Dr. Lily Yang MD, Dr. Zhuo (Georgia) Chen PhD, Dr. Dong M. Shin MD, March/April 2008.
- Nanoparticles for Cancer Therapy: Current Progress and Challenges Shreelaxmi Gavas1, Sameer Quazi2 and Tomasz M. Karpi?ski Gavas et al. Nanoscale Research Letters (2021) 16:173.
- Nanoparticles in cancer therapy and diagnosis Author links open overlay panelIrène Brigger, Catherine Dubernet, Patrick Advanced Drug Delivery Reviews Volume 64, Supplement, December 2012, Pages 24-36.
- Shin WK, Cho J, Kannan A, et al (2016) Cross-linked composite gel polymer electrolyte using mesoporous methacrylate-functionalized SiO2 nanoparticles for lithium-ion polymer batteries. Sci Rep 6:26332.
- Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance Yihan Yao, Yunxiang Zhou, Lihong Liu, Yanyan Xu, Qiang Chen, Yali Wang, Shijie Wu, Yongchuan Deng, Jianmin Zhang and Anwen Shao.
- Nanoparticles for Cancer Therapy: Current Progress and Challenges Shreelaxmi Gavas, Sameer Quazi and Tomasz M. Karpi?ski.
- Novel Tumor-Targeting Nanoparticles for Cancer Treatment - A Review Adelina-Gabriela Niculescu and Alexandru Mihai Grumezescu, Int. J. Mol. Sci. 2022, 23, 5253.
- S. Naahidi, et al. Biocompatibility of engineered nanoparticles for drug delivery J. Control. Release, 166 (2013), pp. 182-194.
- A Recent Review on Cancer Nanomedicine by Paras Mani Giri Anurag Banerjee And Buddhadev Layek, Department of Pharmaceutical Sciences, School of Pharmacy, College of Health Professions, North Dakota State University, Fargo, ND 58105, USA, April 2023.
- Nanoparticles and cancer therapy: A concise review with emphasis on dendrimersDhruba J Bharali, Marianne Khalil, Mujgan GurbuzTessa M SimoneShaker A Mousa.
- Nanoparticles for Cancer Therapy: Current Progress and Challenges, Shreelaxmi Gavas, Sameer Quazi & Tomasz M. Karpi?ski, Volume 16, (2021).
- Lipid-polymer hybrid nanoparticles in cancer therapy: current overview and future directions Francesca Persano, Giuseppe Gigli1, and Stefano Leporatti, 2021, Volume 2.
- Casalini T et al. 2019 A perspective on polylactic acid-based polymers use for nanoparticles synthesis and applications Front. Bioeng. Biotechnol. 7 – 259.
- Mukherjee A et al. 2019 Lipid–polymer hybrid nanoparticles as a next-generation drug delivery platform: state of the art, emerging technologies, and perspectives Int. J. Nanomed. 14, 1937.
- Jing H et al. 2020 Formation and properties of self-assembled nanoparticle-supported lipid bilayer probed through molecular dynamics simulations Langmuir 36 5524–33
- Cell membrane-coated nanomaterials for cancer therapy Author links open overlay panelShiying Zeng a, Qinglai Tang a, Minna Xiao a, Xinying Tong b, Tao Yang a, Danhui Yin a, Lanjie Lei c, Shisheng Li aA, Department of Otorhinolaryngology-Head and Neck Surgery, The Second Xiangya Hospital, Central South University, Changsha, 410011, China, April 2023.
- Cell membrane coated nanoparticles: a novel multifunctional biomimetic drug delivery system Hui Liu, YuYan Su1, XinChi Jiang1, JianQing Gao, 2022.
- Circulating white blood cell traits and colorectal cancer risk: A Mendelian randomization study Andrei-Emil Constantinescu, Caroline J. Bull, Nicholas Jones, Ruth Mitchell, Kimberley Burrows, Niki Dimou, Stéphane Bézieau, Hermann Brenner, Daniel D. Buchanan, 2023.
- Cell membrane-based nanoparticles: a new biomimetic platform for tumor diagnosis and treatment, Ruixiang Li, Yuwei He, Shuya Zhang, Jing Qin, Jianxin Wang, Department of Pharmaceutics, School of Pharmacy, Fudan University, Shanghai 201203, China Key Laboratory of Smart Drug Delivery, Ministry of Education, Shanghai 201203, China Institute of Materia Medica, Academy of Chinese and Western Integrative Medicine, Fudan University, Shanghai 201203, China, 2018.
- Cancer Cell Membrane-Coated Nanoparticles for Anticancer Vaccination and Drug Delivery Ronnie H. FangChe-Ming J. HuBrian T. LukWeiwei GaoJonathan A. CoppYiyin TaiDerek E. O’ConnorLiangfang Zhang.
- Targeting cancer stem cells by using the nanoparticles In-Sun Hong1, Gyu-Beom Jang1, Hwa-Yong Lee Jeong-Seok Nam.
- Nanoparticles for Targeted Drug Delivery to Cancer Stem Cells: A Review of Recent Advances, Yavuz Nuri Ertas, Keyvan Abedi Dorcheh, Ali Akbari and Esmaiel Jabbari.
- Nanoparticle mediated targeting of VEGFR and cancer stem cells for cancer therapy Ambasta et al. Ambasta et al. Vascular Cell 2011, 3:26.
- Sharma, A.; Goyal, A.K.; Rath, G. Recent advances in metal nanoparticles in cancer therapy. J. Drug Target. 2018, 26, 617–632.
- An Overview on Gold Nanorods as Versatile Nanoparticles in Cancer Therapy, Masoud Nejabat, Ali Samie, Mohammad Ramezani, Mona Alibolandi, Khalil Abnous, Seyed Mohammad Taghdisi.
- Gold nanoparticles in cancer therapy, Zhao-Zhin Joanna Lim, Jia-En Jasmine Li, Cheng-Teng Ng, Lin-Yue Lanry Yung & Boon-Huat Bay Acta Pharmacologica Sinica volume 32, pages983–990 (2011).
- Active targeting of gold nanoparticles as cancer therapeutics Zoë Rachael Goddard, María J. Marín, David, Russell and Mark Searcey, October 2020.
- Carbon nanotubes in cancer diagnosis and therapy Shun-rong Ji, Chen Liu, Bo Zhang, Feng Yang, Jin Xu, Jiang Long, Chen Jin, De-liang Fu, Quan-xing Ni, Xian-jun Yu, 2010.
- The application of carbon nanotubes in target drug delivery systems for cancer therapies Nan Volume 6, (2011).
- Zhao, X.; Tian, K.; Zhou, T.; Jia, X.; Li, J.; Liu, P. PEGylated multi-walled carbon nanotubes as a versatile vector for tumor-specific intracellular triggered release with enhanced anti-cancer efficiency: Optimization of length and PEGylation degree. Colloids Surf. B Biointerfaces 2018, 168, 43–49.
- Carbon Nanotubes: An Emerging Drug Carrier for Targeting Cancer Cells, Vaibhav Rastogi, Pragya Yadav, Shiv Sankar Bhattacharya, Arun Kumar Mishra, Navneet Verma, Anurag Verma, Jayanta Kumar Pandit, 2014.
- Exploring the potential of quantum dots as dual modality for cancer therapy and diagnosis Nishant S. Kulkarnia, Yadir Guererrob, Nilesh Gupta, Aaron Mutha, Vivek Gupta, a Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences, St. John’s University, Queens, NY, 11432, USA b Neofluidics Inc., 6650 Lusk Blvd, Suite B102, San Diego, CA, 92121, USA, 2018.
- Quantum dots in cancer therapy Luo, Jiang Long Bo Zhang Chen Liu Shunrong Ji Jin Xu, show all Pages 47-58, Volume 9, 2012 – Issue 1.
- The Research and Applications of Quantum Dots as Nano-Carriers for Targeted Drug Delivery and Cancer Therapy, Volume 11, article number 207, (2016).
- Quantum Dots as a Potential Multifunctional Material for the Enhancement of Clinical Diagnosis Strategies and Cancer Treatments Wenqi Guo1, Xueru Song, Jiaqi Liu, Wanyi Liu, Xiaoyuan Chu, and Zengjie Lei, Nanomaterials 2024, 14, 1088.
- J. Kim, B.T. Huy, K. Sakthivel, H.J. Choi, W.H. Joo, S.K. Shin, M.J. Lee, Y.-I. Lee, Highly fluorescent CdTe quantum dots with reduced cytotoxicity-A Robust biomarker, 1Sens. Bio-Sens. Res. 3 (2015) 46–52.
- Zhu,S.J.; Wang, L.; Li, B.; Song, Y.B.; Zhao, X.H.; Zhang, G.Y.; Zhang, S.T.; Lu, S.; Zhang, J.H.; Wang, H.Y.; et al. Investigation of photoluminescence mechanism of graphene quantum dots and evaluation of their assembly into polymer dots. Carbon 2014, 77, 462–472.
- Recent advancements in mesoporous silica nanoparticles towards therapeutic applications for cancer, Tingting Li, Sixiang Shi, Shreya Goel, Xue Shen, Xiaoxue Xie, Zhongyuan Chen, Hanxi Zhang, Shun Li, Xiang Qin, Hong Yang, Chunhui Wu, Yiyao Liu, 2019.
- Fang, S.; Lin, J.; Li, C.; Huang, P.; Hou, W.; Zhang, C.; Liu, J.; Huang, S.; Luo, Y.; Fan, W. Dual-stimuli responsive nano theranostics for multimodal imaging-guided trimodal synergistic therapy. Small 2017, 13, 1602580.


 Momin M.S.*
Momin M.S.*















 10.5281/zenodo.14175478
10.5281/zenodo.14175478