Abstract
Drug development for the treatment of disease is being revolutionised by RNA-based oligonucleotide therapies. This class of drugs differs from small molecule and protein therapies in several ways, such as how they work and how they are related to clinical pharmacology. Since the COVID-19 mRNA vaccine was approved and nucleoside base alterations won the Nobel Prize in 2023, RNA treatments have gained attention and are revolutionising the drug development process. Although the phrase "RNA therapeutics" has been applied in several settings, the focus of this review is on therapies that target RNA or employ RNA as a component of RNA to achieve therapeutic effects. Small interfering RNA (siRNA) treatments have gained popularity across a variety of therapeutic areas since the first siRNA treatment was licenced in 2018.Thus, it would be beneficial for many parties going forward to do a detailed review of the clinical pharmacology of siRNA therapies with FDA approval. This review covers not only ADME characteristics but also clinical pharmacology data on RNA treatments, such as population pharmacokinetic studies and drug-drug interactions. With the anticipated high growth of the RNA therapies market, thorough understanding will be essential to interpreting and assessing the pharmacological properties.
Keywords
RNA therapeutics, clinical pharmacokinetics, pharmacodynamics, absorption
Introduction
RNA treatments have led the way in several medical applications in the last few times, including vaccinations against the SARS-CoV-2 virus and uncommon illnesses involving gene [removed]COVID-19). The unique target specificity of RNA treatments can offer important advantages over traditional pharmaceuticals by altering the translation of disease-causing proteins by downregulating a gene target or by injecting synthetic mRNA to translate the encoded target protein (kole et al.,2012, Aagaard) (rossi et al.,2007). Given that siRNA was first discovered in 19983, the 2018 licencing of the first short interfering RNA (siRNA) medication, patisiran, highlights the rapid therapeutic advancement in a short period of 20 years(Adams et al.,2018).Two COVID-19 mRNA vaccines were approved for emergency use in 2020 and were administered to millions of patients worldwide, marking an important historical milestone as the first widely utilised RNA medications (Baden et al.,2021) (polack et al.,2020) .On the left is a diagram showing the RNAi pathway and the production of siRNAs. On the right-hand side are various entrance points for RNA- or DNA-based RNAi treatments that penetrate cells and are loaded into RISC to encourage homology-dependent destruction of target mRNA. Viral or non-viral vectors that encode Pol II-driven miRNA mimics or Pol III-driven shRNA can be used to deliver DNA-based therapeutics. RNA interference (RNAi)-based therapeutics can be delivered in a number of forms, including complexed in gold, lipid, or polymer nanoparticles; alternatively, they can be given as synthesised or GalNAc-conjugated dier-substrate siRNA or modified or unmodified siRNA. In mammalian cells, 2 nt 30 overhangs may cause RNAi-based PTGS (Elbashir et al.,2001) (sm et al.,

Figure 1. The RNAi mechanism and entry points of RNAi therapeutics (corydon et al.,2023)
In 2004, the first clinical trial employing siRNA-based drug siRNA-027 was initiated for the treatment of age-related macular degeneration (AMD) (kaiser et al.,2010) (corydon et al.,2023a).
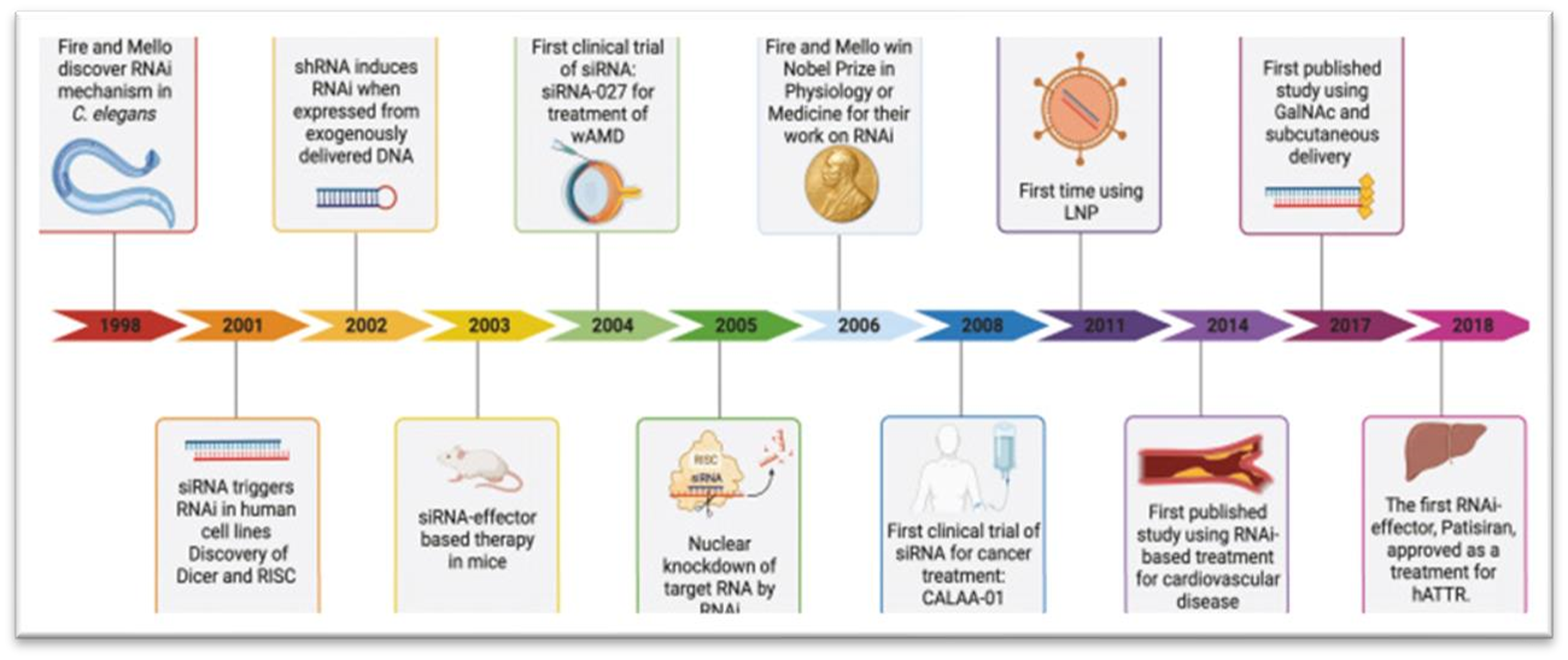
Figure 2: Development of RNAi-based: Milestones: therapeutics (corydon et al.,2023b).
CLASSIFICATION
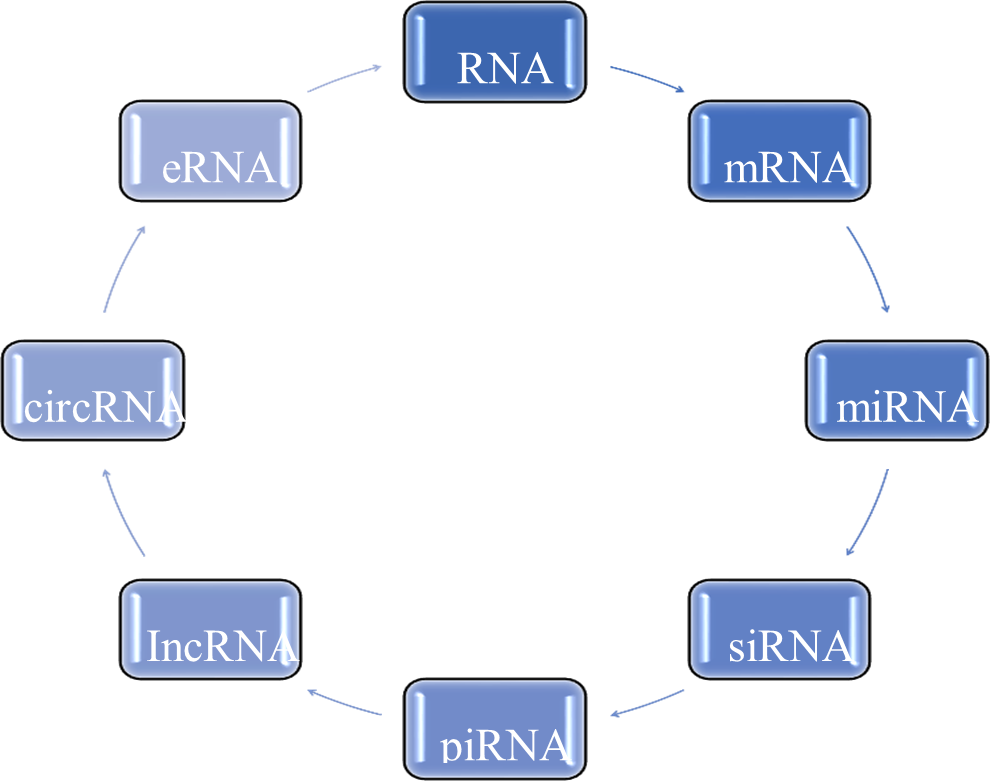
Figure 3: Classification of m-RNA
CODING RNA- mRNA [messenger RNA]
Since it was originally discovered that mRNA functions as templates for the translation of proteins, their biological relevance has long been understood. RNA polymerase is normally used to transcribe pre-mRNA (cole et al., 2001).
SMALL CODIND RNA
miRNA and siRNA
Non-coding RNAs (non-coding siRNAs) like miRNA and siRNA are mostly studied for their capacity to control gene expression, mainly via silencing genes. Their length varies between 20 and 35 nucleotides. While miRNAs are endogenous and expressed from an organism's DNA, siRNAs are widely thought to be mostly foreign in origin, originating from viruses, transposons, or transgenic triggers (rw et al.,2009). The degree of complementarity between the sequences determines the regulatory action that follows target binding. Usually, siRNA drives RISC to precisely matched targets and causes target destruction (ryther et al.,2005). miRNA often interacts with the 3' untranslated region (UTR) of mRNA, occasionally causing different bulges and mismatches. miRNA nucleotides 2–8 represent the seed region, which is necessary for target base pairing and identification (rw et al.,2009a).
PiRNA
With a length of 21–35 nucleotides, piRNA is a kind of short non-coding RNA that is exclusive to animals. Its main functions include regulating gene expression, suppressing transposable elements, particularly in the germline, and preventing viral infection (ozata et al.,2019). Piwi clusters are unique genomic loci where piRNA-encoding genes are found.Pol II transcribes these genes to yield more than 90% of the single-stranded primary piRNAs(chen et al.,2021).
LONG NONCODING RNA [IncRNA]
Longer than 200 nucleotides are the normal length of lncRNAs, which set them apart from smaller ncRNAs like tRNA and miRNA. They can be spliced and polyadenylated similarly to mRNA transcripts, albeit it is not required. Their primary transcription is carried out by RNA pol II (mattick et al.,2023).
Circular RNA [circRNA]
A kind of lncRNA known as "circRNA" has a covalently enclosed loop structure that gives it intrinsic resistance to exonuclease degradation and more stability than linear RNA (enuka et al.,2016; Zhang et al.,2016).
Enhancer RNA [eRNA]
Global profiling and annotation indicate that 40,000–65,000 eRNAs are expressed in human cells (Andersson et al.,2014) (arner et al.,2015) (meers et al.,2018) (chen et al.,2013).
Absorption and Administration Routes
Parenterally administered RNA treatments are frequently administered intravenously or subcutaneously. Because of nucleolytic degradation, RNA treatments have low to moderate bioavailability after subcutaneous delivery when compared to other drugs (ovacik et al.,2018) (keizer et al.,2010). For instance, it was shown that inclisiran's systemic bioavailability in rats was 35.1–48.9% and in monkeys, 24.7–33.8%, following subcutaneous treatment(EMA et al.,2022).
Distribution
In the cytoplasm or nucleus of cells, RNA therapies interact with their RNA targets (Liberman et al.,2018). As the primary excretion and metabolic organs, the kidney and liver receive a systemic distribution of most RNA therapies(amantana and lversen et al.,2005)(sands et al.,1994)(rosie et al.,2004).
Metabolism:
Exo- and endonucleases are typical mediators of the degradation of RNA therapies. Generally speaking, 3-exonucleases break down unmodified oligonucleotides quickly (EDER et al.,1991) (wojcik et al.,2007) (dirin and winkler et al.,2013). Since the liver is where most drug processing occurs, medicines are mostly transported to this organ out of all the others. Certain RNA therapeutics have been examined using recombinant cytochrome P450 (CYP) systems, liver microsomes, or the s9 part of the liver to better understand how each medicine is metabolised. Clinical interactions with CYP enzymes are uncommon because the majority of GalNAc siRNA medicines are not CYP enzyme substrates (Ramsden et al.,2019).
Elimination
Each RNA therapy has distinct excretion-related pharmacokinetic characteristics, even though renal clearance accounts for the majority of RNA treatments' primary excretion. For example, the half-life of fomivirsen, which is cleared from the vitreous humour at a first-order clearance rate, is around 55 hours. Folimivirsen levels in the vitreous humour may decrease over time due to absorption into the retina and other ocular tissues or nucleases metabolising the compound (geary et al.,2002). In addition, during the first 24 hours after a single intake, mipomersen excretes relatively little in the urine. People specifically excrete 1.38 to 3.30% throughout the course of the first 24 hours following a 2-hour intravenous infusion (bell et al ,. 2011).Research utilising the 5/6 nephrectomy rat model, which symbolises moderate to severe renal impairment, revealed that neither the liver's PK profile nor the outcomes associated with Parkinson's disease were significantly affected by a decrease in urine production(mcdougall et al.,2022).
Pharmacokinetic-Pharmacodynamic PK/PD Relationships
By creating a link between dose-concentration relationships (pharmacokinetics, PK) and concentration-effect relationships (pharmacodynamics, PD), PK/PD analyses calculate the effect of pharmaceutical doses across time (derendorf and meibohm et al.,1999). Although PD markers for RNA therapeutics are well-established, the PK/PD connection is more complex than it is for conventional small drugs. Additionally, biologics display the intricate PK/PD interaction, which was clarified by the target-mediated drug disposal mechanism (an et al.,2020) Alternative PK profiles need to be identified in order to resolve this difference in the plasma profile with PD activity. It was found that PD activity is more closely correlated with drug concentration in organs than plasma concentration in cases where RNA treatments target specific organs (Shimizu et L.,2015). Since RISC is a structural prerequisite for the activity of siRNA medicines, PD effect profiles in nonclinical trials substantially more closely match RISC concentration (mcdougall et al.,2022a).
CONCLUSION
The use of SiRNAs as therapeutic agents has changed the landscape of drug development. An extensive assessment of the distinct clinical pharmacology, safety, and efficacy characteristics of this new class of medicines is being actively sought after. The FDA-approved siRNA treatments have distinct clinical pharmacology features when compared to small compounds and protein-based medicines, as this study explains. Due to miRNA's special ability to target numerous mRNA transcripts, a single medication can now regulate several targets or biochemical pathways—many of which are dysregulated in disease. To do this, thorough basic and clinical research is required to address aspects including dose, cross-reactivity, on-target efficacy, and undesired effects before they can receive regulatory approval. Numerous RNA therapies have been developed to treat a variety of ailments, and their approval for use in treating a range of diseases is a result of the encouraging outcomes of numerous clinical trials. But the main obstacle to the development of RNA therapies for many diseases is the absence of appropriate delivery mechanisms.
REFERENCE
- Kole R, Krainer AR, Altman S. RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nature reviews Drug discovery. 2012 Feb;11(2):125-40.
- Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Advanced drug delivery reviews. 2007 Mar 30;59(2-3):75-86.
- Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang CC, Ueda M, Kristen AV, Tournev I, Schmidt HH, Coelho T, Berk JL, Lin KP. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. New england journal of medicine. 2018 Jul 5;379(1):11-21.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. New England journal of medicine. 2021 Feb 4;384(5):403-16.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New England journal of medicine. 2020 Dec 31;383(27):2603-15.
- Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21-and 22-nucleotide RNAs. Genes & development. 2001 Jan 15;15(2):188-200.
- Sm E. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494-8.
- Corydon IJ, Fabian-Jessing BK, Jakobsen TS, Jørgensen AC, Jensen EG, Askou AL, Aagaard L, Corydon TJ. 25 years of maturation: A systematic review of RNAi in the clinic. Molecular Therapy-Nucleic Acids. 2023 Sep 9.
- Kaiser PK, Symons RA, Shah SM, Quinlan EJ, Tabandeh H, Do DV, Reisen G, Lockridge JA, Short B, Guerciolini R, Nguyen QD. RNAi-based treatment for neovascular age-related macular degeneration by Sirna-027. American journal of ophthalmology. 2010 Jul 1;150(1):33-9.
- Cole CN. Choreographing mRNA biogenesis. nature genetics. 2001 Sep 1;29(1):6-7.
- Rw C. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642-55.
- Ryther RC, Flynt AS, Phillips J, Patton JG. siRNA therapeutics: big potential from small RNAs. Gene therapy. 2005 Jan;12(1):5-11.
- Ozata DM, Gainetdinov I, Zoch A, O’Carroll D, Zamore PD. PIWI-interacting RNAs: small RNAs with big functions. Nature Reviews Genetics. 2019 Feb;20(2):89-108.
- Chen S, Ben S, Xin J, Li S, Zheng R, Wang H, Fan L, Du M, Zhang Z, Wang M. The biogenesis and biological function of PIWI-interacting RNA in cancer. Journal of hematology & oncology. 2021 Jun 12;14(1):93.
- Mattick JS, Amaral PP, Carninci P, Carpenter S, Chang HY, Chen LL, Chen R, Dean C, Dinger ME, Fitzgerald KA, Gingeras TR. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nature reviews Molecular cell biology. 2023 Jun;24(6):430-47.
- Enuka Y, Lauriola M, Feldman ME, Sas-Chen A, Ulitsky I, Yarden Y. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic acids research. 2016 Feb 18;44(3):1370-83.
- Zhang Y, Xue W, Li X, Zhang J, Chen S, Zhang JL, Yang L, Chen LL. The biogenesis of nascent circular RNAs. Cell reports. 2016 Apr 19;15(3):611-24.
- Kristensen LS, Andersen MS, Stagsted LV, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nature reviews genetics. 2019 Nov;20(11):675-91.
- Andersson R, Gebhard C, Miguel-Escalada I, Hoof I, Bornholdt J, Boyd M, Chen Y, Zhao X, Schmidl C, Suzuki T, Ntini E. An atlas of active enhancers across human cell types and tissues. Nature. 2014 Mar 27;507(7493):455-61.
- Arner E, Daub CO, Vitting-Seerup K, Andersson R, Lilje B, Drabløs F, Lennartsson A, Rönnerblad M, Hrydziuszko O, Vitezic M, Freeman TC. Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science. 2015 Feb 27;347(6225):1010-4.
- Meers MP, Adelman K, Duronio RJ, Strahl BD, McKay DJ, Matera AG. Transcription start site profiling uncovers divergent transcription and enhancer-associated RNAs in Drosophila melanogaster. BMC genomics. 2018 Dec;19:1-20.
- Chen RA, Down TA, Stempor P, Chen QB, Egelhofer TA, Hillier LW, Jeffers TE, Ahringer J. The landscape of RNA polymerase II transcription initiation in C. elegans reveals promoter and enhancer architectures. Genome research. 2013 Aug 1;23(8):1339-47.
- Ovacik M, Lin K. Tutorial on monoclonal antibody pharmacokinetics and its considerations in early development. Clinical and translational science. 2018 Nov;11(6):540-52.
- Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clinical pharmacokinetics. 2010 Aug;49:493-507.
- EMA EH. CHMP/559383/2017< https>(2017). Accessed April. 2022.
- Lieberman J. Tapping the RNA world for therapeutics. Nature structural & molecular biology. 2018 May;25(5):357-64.
- Amantana A, Iversen PL. Pharmacokinetics and biodistribution of phosphorodiamidate morpholino antisense oligomers. Current opinion in pharmacology. 2005 Oct 1;5(5):550-5.
- Sands H, Gorey-Feret LJ, Cocuzza AJ, Hobbs FW, Chidester D, Trainor GL. Biodistribution and metabolism of internally 3H-labeled oligonucleotides. I. Comparison of a phosphodiester and a phosphorothioate. Molecular pharmacology. 1994 May 1;45(5):932-43.
- Rosie ZY, Geary RS, Monteith DK, Matson J, Truong L, Fitchett J, Levin AA. Tissue disposition of 2??O?(2?methoxy) ethyl modified antisense oligonucleotides in monkeys. Journal of pharmaceutical sciences. 2004 Jan 1;93(1):48-59.
- EDER PS, DeVINE RJ, DAGLE JM, WALDER JA. Substrate specificity and kinetics of degradation of antisense oligonucleotides by a 3? exonuclease in plasma. Antisense research and development. 1991;1(2):141-51.
- Wójcik M, Cie?lak M, Stec WJ, Goding JW, Kozio?kiewicz M. Nucleotide pyrophosphatase/phosphodiesterase 1 is responsible for degradation of antisense phosphorothioate oligonucleotides. Oligonucleotides. 2007 Mar 1;17(1):134-45.
- Dirin M, Winkler J. Influence of diverse chemical modifications on the ADME characteristics and toxicology of antisense oligonucleotides. Expert opinion on biological therapy. 2013 Jun 1;13(6):875-88.
- Ramsden D, Wu JT, Zerler B, Iqbal S, Jiang J, Clausen V, Aluri K, Gu Y, Dennin S, Kim J, Chong S. In vitro drug-drug interaction evaluation of GalNAc conjugated siRNAs against CYP450 enzymes and transporters. Drug Metabolism and Disposition. 2019 Oct 1;47(10):1183-94.
- Geary RS, Henry SP, Grillone LR. Fomivirsen: clinical pharmacology and potential drug interactions. Clinical pharmacokinetics. 2002 Apr;41:255-60.
- Bell DA, Hooper AJ, Burnett JR. Mipomersen, an antisense apolipoprotein B synthesis inhibitor. Expert opinion on investigational drugs. 2011 Feb 1;20(2):265-72.
- McDougall R, Ramsden D, Agarwal S, Agarwal S, Aluri K, Arciprete M, Brown C, Castellanos-Rizaldos E, Charisse K, Chong S, Cichocki J. The nonclinical disposition and pharmacokinetic/pharmacodynamic properties of N-Acetylgalactosamine–conjugated small interfering RNA are highly predictable and build confidence in translation to human. Drug Metabolism and Disposition. 2022 Jun 1;50(6):781-97.
- Derendorf H, Meibohm B. Modeling of pharmacokinetic/pharmacodynamic (PK/PD) relationships: concepts and perspectives. Pharmaceutical research. 1999 Feb;16:176-85.
- An G. Concept of pharmacologic target?mediated drug disposition in large?molecule and small?molecule compounds. The Journal of Clinical Pharmacology. 2020 Feb;60(2):149-63.
- Shimizu R, Kitade M, Kobayashi T, Hori SI, Watanabe A. Pharmacokinetic–pharmacodynamic modeling for reduction of hepatic apolipoprotein B mRNA and plasma total cholesterol after administration of antisense oligonucleotide in mice. Journal of pharmacokinetics and pharmacodynamics. 2015 Feb;42:67-77.


 Mayuri Jagtap*
Mayuri Jagtap*



 10.5281/zenodo.13884776
10.5281/zenodo.13884776