Artificial intelligence (AI) is revolutionizing the field of gene therapy by enabling more precise, efficient, and personalized treatments. AI tools, particularly machine learning (ML) and deep learning (DL) algorithms, are being leveraged to analyze large genomic datasets, identify potential gene targets, and predict the outcomes of genetic modifications. These AI-driven approaches can optimize gene editing techniques like CRISPR-Cas9 by predicting off-target effects and improving editing accuracy. Moreover, AI enhances the design of gene delivery systems, aiding in the development of safer and more effective vectors for gene transfer. In the realm of personalized medicine, AI is crucial in identifying patient-specific genetic variations that can influence treatment outcomes, enabling tailored therapies. Additionally, AI models are being used to monitor and assess long-term effects of gene therapy, allowing for adaptive strategies and real-time adjustments in clinical trials. Despite challenges, such as data quality and algorithm transparency, the integration of AI into gene therapy holds promise for accelerating the development of groundbreaking treatments for genetic disorders, offering new hope for precision medicine. As AI continues to evolve, its role in gene therapy will likely become increasingly integral to transforming medical practice.
Artificial Intelligence,Gene therapy,Gene Editing,Machine learning, Deep learning.
This introduction sets the stage for a comprehensive discussion about the impact of AI in gene therapy, touching upon its history, applications, challenges, and future directions.
Gene therapy aims to treat or prevent disease by modifying genetic material within a patient's cells. With the rapid advancement of AI technologies, there is growing interest in their potential to revolutionize gene therapy. AI capabilities in data analysis, pattern recognition, and predictive modelling offer promising enhancement to traditional gene therapy approaches. This article explores how AI is impacting gene therapy and outlines both advancements and challenges associated with this integration. The intersection of artificial intelligence (AI) and gene therapy represents a transformative frontier in medical science. Recent advancements leverage AI to enhance the precision and efficacy of gene editing techniques, promising revolutionary changes in the treatment of genetic disorders. This review examines a recent article that explores the multifaceted impact of AI on gene therapy, focusing on its applications, benefits, and potential challenges. The emergence of artificial intelligence (AI) has heralded a new era in various fields, including healthcare and biotechnology. One of the most promising applications of AI is in gene therapy, a revolutionary approach that aims to treat or prevent disease by directly altering the genetic material within a patient’s cells. Gene therapy holds the potential to address a wide range of genetic disorders, from rare inherited conditions to more common diseases like cancer. However, the complexity of the human genome and the intricacies of gene editing present significant challenges. Here, AI steps in as a transformative ally, enhancing the efficacy, safety, and personalization of gene therapy approaches.
Artificial intelligence (AI) has increasingly become a powerful tool in advancing various fields, including healthcare. Gene therapy, a cutting-edge approach to treating genetic disorders, is one area where the integration of AI is poised to revolutionize the way we understand and apply genetic interventions. The marriage of AI and gene therapy holds immense promise in enabling personalized treatment strategies, improving patient outcomes, and accelerating research and development in the field.
Figure: 1
One of the primary ways AI is impacting gene therapy is through its ability to analyze vast amounts of genetic data quickly and accurately. By leveraging machine learning algorithms, AI can identify patterns and correlations within genetic information that would be challenging, if not impossible, for human researchers to detect. This enables a more comprehensive understanding of the genetic basis of various diseases and can lead to the discovery of novel targets for gene therapy interventions.
AI also plays a crucial role in optimizing the design of gene therapy vectors, which are vehicles used to deliver therapeutic genes into target cells. Through computational modeling and predictive algorithms, AI can help researchers tailor these vectors for maximum efficiency and specificity, reducing off-target effects and improving the overall safety and efficacy of gene therapy treatments.
Furthermore, AI-powered platforms can assist in predicting patient responses to gene therapy interventions by analyzing genetic variations and other relevant clinical data. This personalized approach allows for the customization of treatment strategies based on individual genetic profiles, leading to more precise and effective therapeutic outcomes.
In addition to enhancing the clinical implementation of gene therapy, AI is accelerating the drug discovery and development process by streamlining the identification of potential gene targets and optimizing the design of gene editing tools. By analyzing vast datasets and predicting the outcomes of genetic modifications, AI can significantly reduce the time and cost associated with bringing new gene therapies to market, ultimately benefiting patients in need of these innovative treatments.
Despite these advancements, challenges remain in integrating AI into gene therapy practices, such as the need for robust data privacy and security measures, ensuring the transparency and interpretability of AI algorithms, and addressing ethical considerations related to the use of AI in genetic manipulation. However, with ongoing research and collaboration among scientists, clinicians, and AI experts, the potential of AI in revolutionizing gene therapy holds great promise for the future of precision medicine.
GENETIC DISORDERS
Genetic disorders are a group of conditions caused by changes or mutations in an individual’s genes. These mutations can be inherited from one or both parents or can occur spontaneously during a person’s lifetime. Genetic disorders can affect any part of the body and can vary widely in how they impact an individual’s health and quality of life.
Understanding Genetic Disorders
Genetic disorders arise from variations in an individual’s DNA. These variations can affect protein function, leading to a range of diseases. Understanding these disorders is crucial for developing effective therapies.
Single-gene disorders:
These disorders are caused by mutations in a single gene, such as cystic fibrosis, sickle cell anemia, and Huntington’s disease.
Multifactorial disorders:
These disorders involve multiple genes and environmental factors. Examples include heart disease, diabetes, and cancer.
Chromosomal disorders:
These disorders result from alterations in the number or structure of chromosomes, such as Down syndrome and Turner syndrome.
There are thousands of known genetic disorders, each with its own set of symptoms and severity. Some genetic disorders are relatively mild and may have minimal impact on a person’s daily life, while others can be severe or even life-threatening. Common examples of genetic disorders include Down syndrome, cystic fibrosis, sickle cell anemia, and Huntington’s disease.
Symptoms of genetic disorders can range from physical abnormalities, such as facial features or limb deformities, to developmental delays, intellectual disabilities, and susceptibility to certain diseases. Diagnosis of genetic disorders often involves a combination of medical history, physical examination, and genetic testing. Genetic counseling may also be recommended to help individuals and families understand the implications of a genetic disorder and make informed decisions about treatment and management options.
Treatment of genetic disorders varies depending on the specific condition. Some genetic disorders have no cure and require ongoing medical care to manage symptoms and complications. In other cases, treatment may include medications, surgery, physical therapy, or other interventions to improve quality of life and reduce the impact of the disorder on an individual’s health.
Advances in genetics research have led to a better understanding of genetic disorders and the development of new treatment options. Genetic testing and screening are becoming more widely available, allowing healthcare providers to identify individuals who may be at risk for certain genetic conditions and provide personalized care and preventive measures.
As the field of genetics continues to evolve, there is hope that ongoing research will lead to new treatments and even potential cures for genetic disorders. In the meantime, raising awareness about genetic disorders, promoting genetic testing and counseling, and supporting individuals and families affected by these conditions are important steps in improving the lives of those living with genetic disorders.
Gene Therapy
Gene therapy is a technique that modifies a person’s genes to treat or prevent disease[Fig: 2]. By introducing, removing, or altering genetic material within a patient’s cells, gene therapy aims to correct genetic disorders at their source. This innovative approach can address conditions caused by single-gene mutations, chromosomal abnormalities, or even multifactorial diseases.
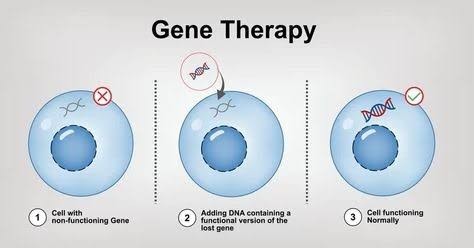
Figure: 2
Types of Gene Therapy
Gene therapy can be classified into several types based on the target cells and the methods used:
Definition: Involves modifying the genes in somatic (non-reproductive) cells.
Applications:
Used to treat conditions such as cystic fibrosis, muscular dystrophy, and certain cancers.
Mechanism:
Typically involves the delivery of a healthy copy of a gene to affected tissues.
Definition: Involves altering the genes in reproductive cells (sperm or eggs).
Applications: This approach is still largely experimental and raises significant ethical considerations. It could potentially eliminate hereditary diseases from a family line.
Mechanism:Changes made to germline cells can be passed on to future generations.
CRISPR-Cas9: A revolutionary tool that allows for precise modifications of DNA sequences. It is widely used for its efficiency and versatility.
Zinc Finger Nucleases (ZFNs): Engineered proteins that can target specific DNA sequences for modification.
Transcription Activator-Like Effector Nucleases (TALENs): Similar to ZFNs, these are proteins that bind to specific DNA sequences to edit genes.
- Viral Vector-Mediated Gene Therapy
Definition: Uses modified viruses to deliver therapeutic genes into target cells.
Types of Vectors: Common viral vectors include retroviruses, adenoviruses, and lentiviruses, each with specific advantages and limitations.
Definition: Involves the use of physical or chemical methods to deliver genes without using viruses.
Methods: Techniques include liposomes, electroporation, and nanoparticle-based delivery systems.
Historical Background
The concept of gene therapy began to take shape in the 1960s with the discovery of the structure of DNA and the understanding of how genes function. Key milestones in the history of gene therapy include:
- Early Research (1960s-1980s)
Discovery of DNA structure (Watson and Crick, 1953).
Early attempts at gene therapy focused on understanding genetic diseases.
- First Human Gene Therapy Trial (1990)
Conducted in the U.S. on a four-year-old girl with severe combined immunodeficiency (SCID). Researchers inserted a functional gene into her cells.
- Establishment of Regulatory Frameworks (1990s)
The development of ethical guidelines and regulatory standards for gene therapy research and clinical trials.
- Gene Therapy for Genetic Disorders (2000s)
Advancements in vector technology and gene editing tools led to successful treatments for various genetic disorders, including Leber’s congenital amaurosis and certain hemophilias.
The introduction of CRISPR-Cas9 transformed gene editing, enabling precise and efficient modifications to the genome.
Milestones
- 2000s: First approved gene therapy products for treating genetic disorders such as Leber’s congenital amaurosis.
- 2017: The FDA approved Luxturna, a gene therapy for inherited retinal disease, marking a significant achievement.
- 2020: Zolgensma received approval for spinal muscular atrophy, illustrating the potential for one-time gene therapies to provide lasting benefits.
Current Applications in Medicine
Gene therapy is currently being applied in various areas of medicine, demonstrating its potential across multiple domains:
Application: Gene therapies targeting cancer cells to induce apoptosis or enhance immune responses.
Example: CAR-T cell therapy, where patients’ T cells are genetically modified to better recognize and attack cancer cells.
Application: Treating hereditary diseases by correcting or replacing defective genes.
Example: Luxturna for retinal dystrophy, which involves delivering a correct copy of the RPE65 gene.
Application: Developing gene therapies to combat viral infections.
Example: Research into gene therapies aimed at HIV and hepatitis C, focusing on modifying immune responses.
Application: Targeting conditions that lack effective treatments due to their rarity.
Example: Zolgensma, a gene therapy for spinal muscular atrophy (SMA), delivering a functional copy of the SMN1 gene.
Application: Gene therapy is being explored for tissue regeneration and repair.
Example: Strategies to regenerate heart tissue after myocardial infarction through gene delivery.
The Rise of Gene Therapy
Gene therapy has evolved significantly since its inception in the late 20th century. Early experiments focused on simple gene replacement strategies, primarily using viral vectors to deliver therapeutic genes into patients’ cells. Over the years, advances in genetic engineering, particularly the development of CRISPR-Cas9 technology, have revolutionized the field. This gene-editing tool allows for precise modifications to the genome, enabling researchers to delete, insert, or alter specific DNA sequences with unprecedented accuracy. Despite these advancements, the field still grapples with substantial obstacles, including off-target effects, delivery challenges, and the need for thorough understanding of complex genetic interactions.
The Role of Artificial Intelligence
AI encompasses a range of computational techniques designed to mimic human cognitive functions, such as learning, reasoning, and problem-solving. In the context of gene therapy, AI algorithms can process vast amounts of genetic data, identify patterns, and generate predictions, thereby facilitating the design and implementation of gene therapies. Machine learning, a subset of AI, is particularly valuable in recognizing complex relationships within large datasets, providing insights that would be difficult for human researchers to discern.
One of the key advantages of AI is its ability to analyze diverse data types—from genomic sequences to clinical outcomes—integrating this information to support decision-making. As gene therapy progresses toward personalized medicine, the role of AI becomes increasingly crucial. By analyzing individual patient data, AI can help tailor gene therapies to meet specific needs, ultimately improving treatment efficacy and minimizing adverse effects.
The Role of Artificial Intelligence in Gene Therapy
Artificial intelligence (AI) refers to the simulation of human intelligence processes by computer systems, enabling machines to perform tasks that typically require human intelligence. In the context of gene therapy, AI enhances the efficiency and effectiveness of various processes, including gene discovery, target identification, treatment personalization, and data analysis.
Machine Learning
Machine learning (ML) is a subset of AI that focuses on developing algorithms that allow computers to learn from and make predictions based on data. In gene therapy, machine learning is employed to:
Identify Genetic Patterns:
By analyzing vast datasets of genetic information, ML algorithms can uncover correlations between specific gene mutations and diseases.
Predict Outcomes:
ML models can predict the efficacy of gene therapies based on patient-specific data, helping to tailor treatments.
Deep Learning
Deep learning is a more advanced branch of machine learning that utilizes neural networks with multiple layers (deep neural networks) to process complex data patterns. In gene therapy, deep learning plays a crucial role in:
Image Analysis:
Analyzing images from gene sequencing and diagnostics to identify genetic abnormalities.
Genomic Data Interpretation: Processing large volumes of genomic data to identify mutations and suggest potential therapeutic targets.
AI Technologies Relevant to Gene Therapy
Genomic Data Analysis Tools
AI technologies facilitate the analysis of complex genomic datasets, enabling researchers to:
Genotype-Phenotype Mapping: Machine learning models can correlate genetic variations with observable traits, aiding in disease understanding and treatment selection.
Variant Prioritization: Algorithms can rank genetic variants based on their potential impact on disease, streamlining the drug discovery process.
Predictive Modeling
AI-driven predictive models are instrumental in anticipating patient responses to gene therapies. These models consider:
Patient Genetic Background: Incorporating individual genomic data to forecast therapeutic outcomes.
Treatment Efficacy: Evaluating past clinical trial data to inform current treatment strategies.
Natural Language Processing (NLP)
NLP, a branch of AI, enables the extraction of relevant information from scientific literature, clinical notes, and patient records. This capability assists in:
Knowledge Discovery: Summarizing existing research findings to identify potential therapeutic targets. Clinical Decision Support: Providing insights to clinicians based on up-to-date literature and treatment guidelines.
Drug Discovery Platforms
AI-powered platforms are transforming drug discovery in gene therapy by:
Identifying New Targets: Using algorithms to analyze biological data and discover novel genetic targets for therapy.
Optimizing Drug Design: Machine learning aids in designing more effective delivery systems for gene therapies.
Clinical Trial Optimization
AI technologies enhance the design and execution of clinical trials by:
Patient Recruitment: Using predictive analytics to identify suitable candidates for trials based on genetic and clinical profiles.
Monitoring Outcomes: Employing AI to analyze real-time data from trials, allowing for adaptive trial designs that can modify protocols based on interim results.
Innovative and Promising: A Comprehensive Overview of Gene Therapy
Gene therapy is a cutting-edge biomedical technique that holds great promise for revolutionizing medical treatment by targeting the root causes of genetic disorders. This innovative approach involves introducing genetic material into a patient’s cells to correct genetic abnormalities or provide therapeutic benefits. With the potential to treat a wide range of diseases, gene therapy offers new hope for patients with genetic disorders that were previously considered untreatable.
The concept of gene therapy first emerged in the 1970s, and since then, significant progress has been made in the field. The development of advanced technologies, such as viral vectors and gene editing tools like CRISPR-Cas9, has facilitated the delivery of therapeutic genes into target cells with precision and efficiency. These tools have transformed gene therapy from a theoretical concept into a practical treatment strategy with tangible clinical applications.
One of the key advantages of gene therapy is its ability to target the underlying genetic cause of a disease, rather than just managing the symptoms. By introducing functional genes into a patient’s cells, gene therapy has the potential to correct genetic mutations, restore normal gene function, and halt the progression of genetic disorders. This targeted approach offers new possibilities for treating rare genetic diseases, such as cystic fibrosis, muscular dystrophy, and hemophilia, as well as more common conditions like certain types of cancer and cardiovascular diseases. Moreover, gene therapy has shown promising results in clinical trials for a variety of conditions, including inherited genetic disorders, autoimmune diseases, and certain types of cancer. For example, gene therapy has been successfully used to treat patients with severe combined immunodeficiency (SCID), a rare genetic disorder that affects the immune system. By introducing a functional copy of the defective gene into the patient’s cells, gene therapy has restored immune function and enabled patients to live normal, healthy lives.
In recent years, the field of gene therapy has witnessed several breakthroughs that have propelled the technology into the spotlight. These advancements include the approval of the first gene therapy products by regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). These approvals have paved the way for the commercialization of gene therapy treatments and have provided new treatment options for patients with genetic disorders. Despite these exciting advancements, gene therapy also faces challenges and limitations that must be addressed. These include concerns about the safety and long-term effects of gene editing technologies, ethical considerations related to the use of gene therapy in humans, and the high cost of developing and manufacturing gene therapy products. Additionally, issues related to the delivery of therapeutic genes to target cells, immune responses to gene therapy vectors, and potential off-target effects of gene editing tools remain areas of active research and development.
Looking ahead, the future of gene therapy holds great promise for advancing personalized medicine and transforming the treatment of genetic disorders. Ongoing research efforts are focused on improving the safety and efficacy of gene therapy techniques, expanding the range of treatable conditions, and addressing the challenges associated with the widespread adoption of gene therapy as a mainstream medical treatment.
Enhancing Gene Targeting and Delivery in Gene Therapy
One of the key challenges in gene therapy is the effective targeting and delivery of therapeutic genes to the appropriate cells. Artificial intelligence (AI) has emerged as a powerful tool to enhance these processes, improving precision and minimizing off-target effects. This section explores how AI algorithms are revolutionizing gene targeting and delivery, along with notable case studies that illustrate these advancements.
AI Algorithms for Targeting Cells
- Machine Learning for Predictive Targeting
Machine learning algorithms analyze vast datasets to predict the most suitable cells for gene delivery. These algorithms can evaluate:
Cell Surface Markers: Identifying specific proteins expressed on the surface of target cells, which can be used for selective targeting.
Tissue Microenvironments: Understanding the local environment of cells to determine the best delivery methods and vectors.
- Reinforcement Learning
Reinforcement learning, a subset of machine learning, involves training algorithms through trial and error. In gene therapy, this approach can optimize delivery strategies by:
Dynamic Adaptation: Adjusting targeting methods in real-time based on feedback from previous delivery attempts.
Exploration of Delivery Routes: Identifying the most effective pathways for gene delivery by exploring various methods and their outcomes.
- Deep Learning for Image Analysis
Deep learning techniques can analyze imaging data (such as microscopy images) to identify and target specific cells. This capability is essential for:
Visualizing Target Cells: Accurately distinguishing target cells from surrounding tissues.
Assessing Delivery Efficiency: Evaluating how well gene therapies are reaching their intended targets.
- Genomic and Proteomic Data Integration
AI algorithms can integrate genomic and proteomic data to:
Identify Patient-Specific Targets: Tailoring gene therapy based on individual genetic profiles to enhance targeting accuracy.
Predict Off-Target Effects: Assessing potential unintended interactions with non-target cells, improving safety.
Case Studies of AI-Enhanced Delivery Systems
- CRISPR Delivery Optimization Using AI
Researchers at the Massachusetts Institute of Technology (MIT) developed a machine learning model to optimize the delivery of CRISPR gene-editing tools. This system analyzed various lipid nanoparticles to determine the most effective formulations for delivering CRISPR components to specific cell types, such as liver and muscle cells. The results demonstrated significantly improved delivery efficiency and reduced off-target effects compared to traditional methods.
- AI-Driven Targeting in CAR-T Cell Therapy
In a study conducted by Stanford University, AI algorithms were employed to enhance CAR-T cell therapy for cancer treatment. Researchers used machine learning to analyze patient data and predict which T cells would be most effective in targeting specific tumors. By identifying the optimal T cell populations and refining their engineering, the therapy demonstrated improved patient outcomes in clinical trials.
- Predictive Modeling for Viral Vector Delivery
A collaborative project involving researchers from several institutions utilized predictive modeling to improve the delivery of viral vectors in gene therapy. By employing machine learning to analyze data from prior experiments, they developed a model that predicted the best viral vector for specific types of cells. This approach led to enhanced transduction efficiency and specificity, as demonstrated in trials involving gene therapies for inherited retinal diseases.
- Nanoparticle Delivery Systems
A research team developed an AI-based platform for designing and optimizing nanoparticle delivery systems. By training algorithms on datasets from previous nanoparticle studies, they successfully identified configurations that maximized cellular uptake and gene delivery efficiency. In preclinical models, these optimized nanoparticles significantly outperformed conventional delivery methods.
Types of AI Tools in Gene Therapy
Artificial intelligence tools are transforming gene therapy by enhancing precision, efficiency, and personalization in treatment strategies. Here, we elaborate on the primary types of AI tools used in gene therapy, along with references for further reading.
- Machine Learning Algorithms
Machine learning (ML) algorithms analyze complex genomic datasets to identify patterns and predict outcomes. These algorithms learn from historical data to enhance their predictive capabilities. These tools analyze vast datasets to predict patient responses to gene therapies, optimizing treatment plans.
Applications:
Predicting Patient Responses: ML can forecast how patients will respond to specific gene therapies, allowing for tailored treatment plans.
Outcome Prediction: Algorithms evaluate historical clinical data to anticipate the efficacy of various therapies?.
- Natural Language Processing (NLP)
NLP enables the analysis of human language, allowing computers to extract meaningful information from large volumes of text. NLP systems facilitate the extraction of relevant information from scientific literature, aiding researchers in staying updated with the latest findings.
Applications:
Literature Mining: NLP can sift through scientific papers to identify potential gene therapy targets and methodologies.
Data Extraction: It helps extract relevant data from clinical trial reports, assisting in the design of future studies?.
- Computer Vision
Computer vision technologies allow for the analysis and interpretation of visual data, particularly useful in examining cellular changes post-gene editing. Used in analyzing gene editing outcomes, particularly in CRISPR applications, by processing images of cellular changes.
Applications:
Microscopy Image Analysis: Utilized to assess the effects of gene editing tools like CRISPR on cell morphology.
Automated Quality Control: Ensures precision in gene editing by detecting unintended genetic modifications8.
- Bioinformatics Software
Bioinformatics merges biology and computer science to analyze biological data, often enhanced with AI to improve data interpretation. Integrates AI to identify gene targets and potential off-target effects, enhancing the safety profiles of gene therapies.
Applications:
Genomic Data Analysis: AI tools identify mutations and variations that are potential targets for therapy.
Off-Target Effect Prediction: AI aids in predicting and minimizing off-target effects in gene editing techniques?.
- Reinforcement Learning
Reinforcement learning (RL) focuses on making decisions through trial and error to maximize cumulative rewards, applicable in optimizing gene delivery systems.
Applications:
Optimizing Gene Delivery: RL can refine the parameters of vectors used in gene delivery for improved efficiency.
Adaptive Treatment Strategies: RL supports dynamic treatment adjustments based on real-time patient data?1;?.
- Genetic Network Modeling
Genetic network modeling simulates interactions within biological systems, helping researchers understand the effects of genetic modifications.
Applications:
Pathway Analysis: Identifies critical pathways involved in diseases, guiding therapeutic target selection.
Predicting System Responses: Assesses how changes in gene expression affect cellular behavior?1;?1;.
- Data Integration Platforms
These platforms aggregate various data types to provide holistic insights for researchers and clinicians.
Applications:
Holistic Patient Profiles: AI tools combine genetic and clinical data to create detailed patient profiles for personalized treatment.
Predictive Analytics: They enhance the prediction of treatment responses by correlating multiple datasets?1;?2;.
Gene editing tools: Revolutionizing the Future of Medicine
Gene editing tools have emerged as a revolutionary technology with the potential to transform the landscape of medicine and healthcare. By enabling scientists to make precise changes in the genetic code of living organisms, these tools have opened up new avenues for treating genetic disorders, developing novel therapies, and even creating genetically modified organisms for various applications.
One of the most well-known gene editing tools is CRISPR-Cas9, a system derived from a bacterial defense mechanism that allows researchers to target specific sequences of DNA and make edits with unprecedented precision. CRISPR-Cas9 has quickly become the go-to tool for gene editing due to its simplicity, efficiency, and versatility. In addition to its use in research labs, CRISPR-Cas9 has the potential to revolutionize medical treatments by correcting genetic mutations responsible for diseases such as cystic fibrosis, sickle cell anemia, and muscular dystrophy.
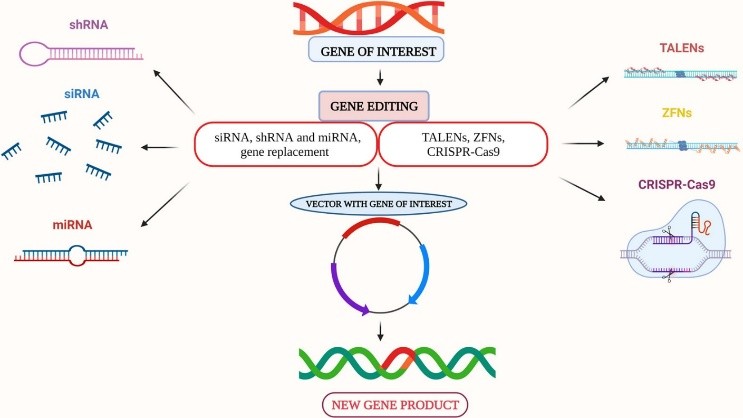
Figure : 3
Another promising gene editing tool is TALENs (transcription activator-like effector nucleases), which operate in a similar manner to CRISPR-Cas9 by targeting specific DNA sequences and inducing changes. TALENs have been utilized in various research studies and have shown great promise in the development of potential gene therapies.
In addition to CRISPR-Cas9 and TALENs, other gene editing technologies such as zinc-finger nucleases (ZFNs) and meganucleases have also been employed by researchers for genetic manipulation. While these tools may not be as widely used as CRISPR-Cas9, they offer unique capabilities and can be valuable in certain applications.
The impact of gene editing tools extends beyond the realm of human health. In agriculture, these tools have the potential to create crops that are resistant to pests, diseases, and environmental stressors, ultimately increasing food security and sustainability. Additionally, gene editing can be used to engineer microbes that produce valuable compounds for industrial applications, such as biofuels and pharmaceuticals.
However, the use of gene editing tools also raises ethical and safety concerns. The potential for unintended consequences, off-target effects, and the creation of genetically modified organisms with unknown risks must be carefully considered and regulated. Additionally, questions surrounding the equitable distribution of gene editing technologies and the ethical implications of editing the human germline remain subjects of ongoing debate and discussion. As gene editing tools continue to advance and evolve, it is essential for scientists, policymakers, and society as a whole to engage in thoughtful dialogue and decision-making to ensure that these powerful technologies are used responsibly and ethically. With careful oversight and responsible application, gene editing tools have the potential to revolutionize medicine, agriculture, and biotechnology, paving the way for a brighter and healthier future.
Mechanisms of AI in Gene Therapy
Artificial intelligence (AI) is revolutionizing the field of gene therapy, offering new mechanisms for improving the precision, efficiency, and safety of gene-based treatments. AI can enhance various stages of gene therapy, from drug discovery and patient selection to therapeutic delivery and monitoring of treatment outcomes. Below are several key mechanisms by which AI is being integrated into gene therapy, supported by relevant research:
AI mechanisms in gene therapy primarily involve:
- Gene Editing Design and Optimization
AI techniques, particularly machine learning (ML) and deep learning (DL), are used to design and optimize gene editing tools like CRISPR/Cas9. These tools can target specific genetic sequences, but the precision of their targeting can sometimes be imprecise, leading to off-target effects. AI can help predict the most effective and safe guide RNA sequences, minimizing the risk of these off-target effects.?1;?3;
- Drug Discovery and Target Identification
AI models are increasingly used to identify potential genetic targets for gene therapy. Through data mining and pattern recognition, AI can analyze large datasets from genomics, proteomics, and transcriptomics to identify novel therapeutic targets. This is particularly useful for genetic diseases that lack well-established targets.?1;?
- Patient Stratification and Personalized Medicine
AI can analyze genomic and clinical data to identify the most suitable candidates for specific gene therapies. By utilizing AI-driven algorithms to classify patients based on genetic variants, biomarkers, and other medical data, clinicians can more accurately predict which patients will benefit most from gene therapy. This personalized approach helps to optimize therapeutic outcomes and reduce the risk of adverse effects.?1;?
- Gene Therapy Delivery Systems
Efficient delivery of genetic material to target cells remains one of the biggest challenges in gene therapy. AI is being employed to design better delivery vectors, such as viral and non-viral systems. By simulating and predicting the behavior of nanoparticles or viral vectors in the human body, AI models can optimize their design, improve targeting efficiency, and minimize immune responses.?1;?
- Real-time Monitoring of Treatment Efficacy
AI-driven tools can be applied to analyze real-time data collected from patients undergoing gene therapy. By integrating data from medical imaging, genomics, and other sensors, AI can help track the progress of the treatment, predict potential complications, and assess the efficacy of the therapy at various stages. This improves decision-making and allows for personalized adjustments to the treatment plan.?1;?
- Predictive Modeling of Genetic Diseases
By Using historical data to predict the efficacy of gene therapies based on patient profiles. AI is also used to develop predictive models of genetic diseases, enabling researchers to simulate how specific mutations may affect cellular processes. By combining genomic data with AI techniques, researchers can predict the progression of genetic disorders, providing valuable insights into the development of gene therapies aimed at halting or reversing disease progression. ?1;?
- Data Mining
Extracting insights from genomic data to identify suitable gene targets.
- Optimization Algorithms
Refining gene delivery systems for enhanced specificity and reduced immunogenicity.
Accelerating Drug Discovery and Development in Gene Therapy
The drug discovery process, traditionally lengthy and expensive, is undergoing a transformation through the integration of artificial intelligence (AI). In the realm of gene therapy, AI plays a pivotal role in identifying gene targets, optimizing candidate selection, and expediting clinical development. This section discusses how AI accelerates drug discovery and presents case studies that exemplify its impact.
1.Data Mining and Pattern Recognition:
AI algorithms can analyze large datasets from genomics, proteomics, and other biological sources to identify potential gene targets for therapy. By recognizing patterns that correlate specific genetic mutations with diseases, AI enhances the efficiency of target identification.
2. Genomic Analysis:
AI techniques such as machine learning and deep learning are employed to analyze genomic sequences, helping researchers identify:
Mutations Associated with Diseases: Algorithms can sift through genetic data to pinpoint variations linked to specific disorders.
Functional Genomics: AI can help in predicting the function of uncharacterized genes based on their sequence similarity to known genes.
3.Biomarker Discovery:
AI facilitates the discovery of biomarkers that can indicate disease progression or response to therapy. By integrating data from clinical trials and patient records, AI can identify novel biomarkers that improve the precision of gene therapies.
Case Studies on AI in Drug Development:
Insilico Medicine and The Discovery of New Drug :
Insilico Medicine utilized deep learning to identify potential drug candidates for fibrosis. By analyzing existing databases of molecular structures and their biological activity, the AI model predicted a novel compound that exhibited therapeutic effects. This compound progressed to preclinical trials in record time, demonstrating how AI can significantly shorten the drug development timeline.?1;?
Bristol-Myers Squibb's AI Driven Target Discovery:
Bristol-Myers Squibb employed AI technologies to streamline target discovery for new cancer therapies. The company used machine learning algorithms to analyze genomic and clinical data, leading to the identification of several novel targets that have advanced into drug development stages.?2;?
Atomwise and – high throughput screening :
Atomwise developed an AI platform that utilizes deep learning for virtual screening of compounds. Their technology can predict how small molecules will interact with specific gene targets. In a notable collaboration with researchers targeting the Ebola virus, Atomwise identified several promising compounds, significantly reducing the time and cost associated with traditional screening methods.?2;?1;
Deep Genomics and Rna based therapeutic :
Deep Genomics harnesses AI to predict the effects of genetic variations on RNA splicing. This capability is critical for developing RNA-targeted gene therapies. Their AI platform has enabled the identification of novel therapeutic targets for various genetic disorders, advancing multiple candidates into preclinical trials.?2;?2;
Data Integration in Gene Therapy:
Data integration in gene therapy involves combining various types of biological, clinical, and genomic data to gain comprehensive insights into treatment efficacy, patient responses, and potential side effects. This can include:
- Genomic Data: Information from DNA sequencing, gene expression profiles, and epigenetic modifications.
- Clinical Data: Patient health records, treatment outcomes, and demographic information.
- Proteomic and Metabolomic Data: Protein expressions and metabolic profiles that can influence therapy responses.
Types of Data in Gene Therapy:
- Genomic Data: Sequence data, variant annotations, and gene regulation patterns.
- Clinical Data: Patient demographics, medical histories, and treatment responses.
- Transcriptomic Data: RNA sequencing data that reveals gene expression levels.
- Proteomic Data: Information on protein expression and interactions.
- Pharmacogenomic Data: Genetic factors that affect drug metabolism and response.
- Phenotypic Data: Observable traits and conditions of patients that can affect therapy outcomes.
Role of Artificial Intelligence in Data Analysis
- Predictive Analytics: AI algorithms can predict patient outcomes based on historical data, helping tailor treatments.
- Data Mining: AI techniques can identify patterns and correlations in large datasets that may not be apparent through traditional analysis.
- Natural Language Processing (NLP): Used to analyze unstructured data from clinical notes and research papers, extracting relevant information for decision-making.
- Machine Learning Models: These can classify and cluster data, identifying subgroups of patients who may respond differently to therapies.
- Optimization of Clinical Trials: AI can enhance trial design by predicting patient recruitment and retention, as well as optimizing dosing strategies.
Examples of Successful Data Integration
- The Cancer Genome Atlas (TCGA): Integrated genomic, transcriptomic, and clinical data to improve understanding of cancer biology and inform treatment strategies.?2;?3;
- NVIDIA Clara Genomics: This platform uses AI to integrate genomic data with imaging and clinical information to support research and clinical applications in genomics.?2;?
- Genentech’s Personalized Healthcare Initiative: Combines genetic data, clinical trials, and patient feedback to optimize drug development and treatment personalization.?2;?
AI & ITS APPLICATIONS IN GENE THERAPY
Artificial intelligence (AI) , and its applications in gene therapy hold great promise for advancing precision medicine and improving patient outcomes. Gene therapy involves altering the genes within an individual’s cells to treat or prevent diseases, offering potential solutions for genetic disorders, cancer, and other health conditions. AI plays a crucial role in enhancing the effectiveness and efficiency of gene therapy by enabling rapid analysis of large volumes of genetic data, predicting treatment outcomes, and personalizing therapies based on individual genetic profiles. Here are some examples of successful applications of AI in gene therapy:
AI models have been instrumental in accelerating the process of drug discovery for gene therapy. By analyzing genetic data and identifying potential drug targets, AI can facilitate the development of novel gene-based therapies for various diseases. For example, AI algorithms have helped researchers identify and design CRISPR-based gene editing tools for precise genetic modifications, leading to groundbreaking advancements in gene therapy.
AI techniques such as machine learning algorithms are being used to analyze vast amounts of genomic and clinical data to tailor gene therapies to individual patients. By considering a patient’s genetic makeup, lifestyle factors, and disease characteristics, AI can predict the most effective treatment strategies and optimize therapeutic outcomes. This personalized approach has been particularly successful in treating cancer through precision oncology, where AI identifies genetic alterations driving tumor growth and suggests targeted gene therapies.
- Disease Diagnosis and Prognosis:
AI-powered tools can aid in the early diagnosis and accurate prognosis of genetic disorders, enabling timely interventions and personalized treatment plans. For instance, AI algorithms can analyze genetic sequencing data to detect specific mutations associated with hereditary diseases and predict disease progression. By leveraging AI in genetic testing and analysis, healthcare practitioners can better understand the underlying genetic mechanisms of diseases and optimize gene therapy strategies accordingly.
- Gene Editing Optimization :
AI algorithms have been applied to optimize gene editing techniques such as CRISPR-Cas9, enhancing their precision and efficiency in modifying specific genes. AI models can predict the off-target effects of gene editing tools and suggest modifications for minimizing unintended genetic alterations. This level of precision ensures the safety and efficacy of gene therapy interventions, paving the way for transformative treatments for genetic diseases.
AI facilitates the design and execution of clinical trials for gene therapy by identifying suitable patient populations, predicting treatment responses, and optimizing trial protocols. AI-driven platforms can analyze diverse datasets to stratify patients based on genetic risk factors and treatment eligibility criteria, enabling more targeted and efficient clinical trials. By leveraging AI in trial design, researchers can accelerate the development and approval of gene therapies, bringing innovative treatments to patients sooner.
The applications listed in Table 1 demonstrate the various and significant applications
Of genetics and AI in the fields of genetics and healthcare. Examples include cancer detection and
Monitoring, identifying at-risk populations, classifying genetic variants, and predicting patient ancestry.


 Vemula Greeshma* 1
Vemula Greeshma* 1
 Dr. C. Bhuvaneswar Rao 2
Dr. C. Bhuvaneswar Rao 2



 10.5281/zenodo.14233536
10.5281/zenodo.14233536