Abstract
One of the leading causes of cancer-related morbidity and mortality among women worldwide is breast cancer, so early detection is crucial to improving survival rates. In order to detect breast cancer early, this review looks at recent advancements in imaging technology, biomarker identification, and artificial intelligence (AI). Newer techniques like computer-aided detection, magnetic resonance imaging, and liquid biopsies aim to overcome the limitations of traditional methods, despite their effectiveness. These developments provide non-invasive disease progression monitoring and improve tumour detection at earlier stages. AI is also transforming the accuracy of diagnosis by enhancing image interpretation and tailoring treatment plans. Patient-centered care and education are crucial to empowering women to actively participate in their own health care. Irrespective potential of these developments, issues like affordability and accessibility still exist, urging for an emphasis on providing healthcare in an appropriate way. The potential of these innovations to revolutionize breast cancer diagnosis is highlighted in this review, that can ultimately result in better patient outcomes and an era where breast cancer is identified earlier and treated more effectively.
Keywords
Breast Cancer, Early Diagnosis, Artificial Intelligence, Computer Aided Detection, Mammography, Genome Analysis.
Introduction
Despite recent tremendous advancements, cancer remains the world's leading cause of death, taking the lives of over 19.3 million people in 2020, or approximately one in six. Breast cancer, the most common disease in women worldwide, is a severe public health issue that requires increased attention and collaborative research [1].
Genetic susceptibility, family history, disease condition, radiation exposure, lifestyle factors (e.g., obesity, physical inactivity, alcohol consumption, diet, smoking, dietary habits), and hormonal and reproductive factors (e.g., early menarche, late menopause, advanced age at first birth, fewer children, less breastfeeding, menopausal hormone therapy, oral contraceptives) are some of the risk factors for breast cancer [2]. It is a multifaceted disease with a range of molecular and clinical characteristics. The primary molecular markers used to differentiate between various subtypes of breast cancer are the presence or absence of the oestrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor 2 (HER2) receptor proteins. Breast cancer (BC) is classified into multiple subgroups based on histological and molecular characteristics, such as Luminal A (ER+, PR+, HER2-), Luminal B (ER+, PR+, HER2+), Normal like HER2+, and TNBC (ER-, PR-, HER2-) [3].
Treatment results are greatly enhanced when breast cancer is detected early. More precise and dependable diagnostic techniques are required in light of the current issues with breast cancer screening, such as false positives and negatives. Since early diagnosis plays a significant role in treatment outcomes and survival rates, its significance cannot be overstated. Breast cancer diagnosis is changing quickly due to research and technological developments [4].
This study explores the most recent advancements in early diagnosis methods, including imaging technology, biomarker detection, and artificial intelligence, and considers how they can affect clinical practice and patient outcomes.
MATERIAL AND METHOD
-
- Approaches to Literature Searches
- Databases: Electronic databases such as PubMed, Embase, Scopus, Google Scholar, Medline, Science Direct, and Frontiers were searched in order to find relevant publications.
- Keywords: "Breast cancer," "early diagnosis of breast cancer," " Mammography," " Computer-Aided Detection," "Future Challenges," and " Liquid Biopsies " were among the pertinent keywords.
- Criteria for Inclusion: studies on breast cancer early detection that have been released in peer-reviewed journals in recent years.
- Criteria for Exclusion: reviews without original data, and articles written in a language other than English.
- Selection of Literature review
- The Screening Procedure: Following a relevance screening of titles and abstracts, full-text reviews were conducted to verify eligibility according to predetermined standards. The reference lists of the included studies, as well as relevant abstracts, conference papers, and reviews of innovative therapeutic approaches, were also reviewed to ensure that all pertinent studies were included.
- Extraction of Data: 250 records in all were found by database searching, and 20 more records were found from other sources. There were 223 records left after 47 duplicates were eliminated. 154 full-text articles were assessed for eligibility after being screened based on abstract and title. Since nine of these were in languages other than English, they were removed, 17 were eliminated because they were not randomized controlled trials (RCTs), and 79 were eliminated because their abstracts showed no connection to one another. In the end, the qualitative-only synthesis contained 110 studies.
- EARLY DETECTION AND DIAGNOSIS OF BREAST CANCER: STRENGTHENING SCREENING INITIATIVES
When breast cancer is detected early and treated, the chances of survival are extremely high. However, a woman's access to prompt, economical, and efficient breast healthcare treatments may be limited by social, economic, geographic, and other interconnected factors. These intricate barriers to early diagnosis present numerous challenges for women [5]. The World Health Organization has developed two distinct but related strategies to promote early cancer detection: early diagnosis, or the early detection of symptomatic cancer, and screening, or the identification of asymptomatic disease in a target population of people who seem to be in good health [6].
Early identification using adjunct approaches in clinical evaluation has drawn interest in recent years as a means of lowering the death rate. Various techniques of breast screening and diagnosis as shown in figure 1.

Figure 1: Various techniques of breast screening and diagnosis
-
- Mammography
Mammography is the main diagnostic technique used to identify breast cancer. A low-dose X-ray system is used in mammography, a form of medical imaging that is primarily used to check for breast cancer [7]. Mammography remains the most important imaging modality for screening because it is the only one that has shown a reduction in mortality. It is categorized into two types Screening and Digital mammography as shown in Figure 2.

Figure 2: Types of Mammography
In the regard of breast cancer screening, mammograms can validate the discovery of a lump or other indication. The procedure is known as a diagnostic mammography. In addition to a lump, other signs of benign diseases include breast soreness, thickening of the breast skin, discharge from the nipples, and changes in the size or shape of the breast. When screening mammography is challenging because of unique conditions, Diagnostic mammography can also be used to examine breast tissue or evaluate changes seen during screening, including breast implants [8,9].
Mammography has a number of drawbacks, such as false positives and negatives that may result in missed cancers or needless procedures. It involves minimal radiation exposure and could be uncomfortable. Women with dense breast tissue may experience decreased effectiveness, and some may find access and cost to be obstacles. Inconsistent diagnoses can also result from differences in interpretation [10].
-
- Computer-Aided Detection (CAD)
Additionally, computer-aided detection (CAD) systems are easier to employ with digital mammograms. These technologies identify patterns in photos that may indicate cancer by using complex computer programs. The CAD system alerts the radiologist if such patterns are found, allowing them to conduct a closer examination of the questionable region. CAD can be used directly for digital mammography or for traditional mammography films that have been digitally transformed [11].
According to several studies, CAD may help radiologists identify and categorize breast abnormalities on mammograms more accurately. According to one study, CAD may have contributed to a nearly three-quarter reduction in the percentage of breast cancers missed during film mammography screening. Further research suggests that the frequency of abnormalities mistakenly reported as possible malignancies (false positives) is not appreciably increased by the inclusion of CAD to mammography screening. However, more thorough research is needed to make sure that CAD doesn't provide any false positive or negative results and to clarify the purpose and proper application of this technology. One CAD detection system for breast cancer screening was just authorized by the FDA [12,13].
-
- Ultrasonography
Ultrasonography uses sound wave methods to provide detailed medical pictures of the breast. Nursing moms and pregnant women unable to undergo CT or X-rays are thought to find this procedure safe and appropriate. Furthermore, women who are expecting or nursing and are unable to utilize CT or X-rays are deemed safe and appropriate candidates for ultrasonography [14,15].
In women with thick breasts, adding ultrasonography screening to mammography improved the number of benign breast biopsies and malignancies found. A large prospective screening trial found that combining ultrasonography and mammography found an additional 4.3 tumors per 1000 cases in women with tight breasts and a high risk of breast cancer. According to reports, the sensitivity of mammography was 50.0% in women with thick breasts, whereas the sensitivity of mammography plus ultrasound was 77.5%. It is recognized as standard practice to use magnetic resonance imaging (MRI) for screening potential in individuals who are genetically predisposed to breast cancer or who have previously received radiation treatment for another cancer. Ultrasound is helpful, but it depends on the operator and might not be as good at screening as mammography. [16,17].
-
- Magnetic Resonance Imaging (MRI)
In order to detect, diagnose, and treat breast cancer, magnetic resonance imaging, or MRI, has become a crucial tool. Because it creates detailed images of breast tissue using radio waves and strong magnetic fields, it is particularly useful in some clinical settings [18]. The sensitivity of contrast-enhanced breast magnetic resonance imaging (MRI) in detecting breast cancer in women at advanced risk for the disease is 90% to 93%, while the combined sensitivity of mammography and ultrasound is 48% to 63%. For those who are genetically predisposed to breast cancer or have had prior radiation treatment for another malignancy, using magnetic resonance imaging (MRI) for detecting and monitoring is accepted as standard of care [19,20]. Breast MRI has been extensively utilized since 2000 and has grown in importance as a tool for high-risk breast cancer screening, diagnosis, staging, and follow-up [21]. Breast magnetic resonance imaging enables high-risk screening, evaluation of an unknown primary disease, assessment of the nearby extent of disease, both bilateral and multicentric, especially in dense breasts, differentiation of a scar from cancer recurrence in women who have undergone breast preservation surgery, and evaluation of the response to neoadjuvant chemotherapy. in addition to the assessment of implant integrity [22].
On the other hand, MRI screening has a higher false-positive rate due to its inferior sensitivity. Although numerous studies have shown the value of MRI screening for early detection in women who are at high risk of breast cancer, such as those with a strong family history or a known genetic predisposition, the data does not support the use of breast MRI as a screening technique for women at average risk. Some countries recommend MRI screening for women who are at higher risk of breast cancer [23,24].
-
- Positron Emission Tomography (PET)
An imaging technique called positron emission tomography (PET) uses a radioactive substance called a tracer to search for potential metastases of breast cancer. Areas of cancer that may not be seen on an MRI or CT scan can be identified using this tracer [25]. F-fluorodeoxyglucose (18F-FDG) is the most frequently used agent for positron emission tomography (PET) imaging. Numerous cancers, including breast cancer, are now routinely diagnosed, staged, and restaged using 18F-FDG in PET/computed tomography (PET/CT). Since breast cancer is linked to higher glucose intake, the glucose analog can be used to visualize the condition [26]. PET can evaluate the entire body for metastases and offers thorough insights, but because of problems like false positives, radiation exposure, and increased costs, it is not frequently used as a primary screening method. Assessing the extent of known breast cancer and tracking treatment outcomes continue to be its most useful uses, helping to provide individualized patient care [27].
-
- Microwave Imaging-Based Technology
In the last ten years, alternative technology has concentrated on research incorporating Microwave Imaging (MI) for breast cancer screening to address the drawbacks, expense, high risk, and inconvenience of X-rays and MRIs. MI is an imaging technique for nonionizing electromagnetic signals that operate in the 300 MHz to 30 GHz frequency range. MI helps identify malignancies by providing greater and more pronounced contrast between healthy tissue and tumors without raising the possibility of ionization effects. Patients are safe because it is non-invasive, which means that neither ionizing radiation nor invasive treatments are used. It is also reasonably priced, which makes it a feasible choice for broad usage [28].
Furthermore, because MI methods are based on the difference in electrical characteristics between normal and tumorous breast tissues, they are more sensitive and capable of detecting tiny breast tumors. For microwave-based breast detection, three modalities have been investigated as seen in Figure 3. These techniques fall into three categories: hybrid, active, and passive [29].
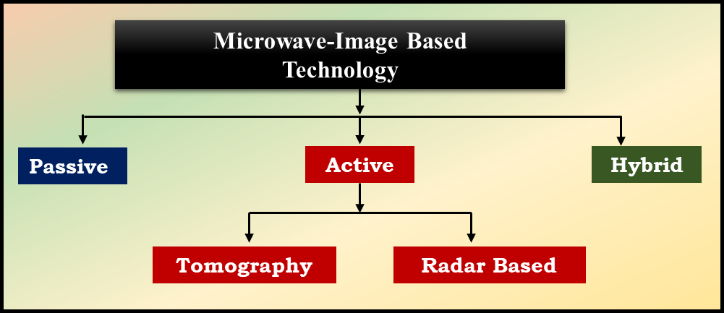
Figure 3: Microwave-Image Based Technology
The passive method uses a radiometry instrument to detect temperature variations between breast cancerous and healthy tissues. Ultrasonic transducers and microwave sensors are used in hybrid approaches, sometimes known as thermoacoustic methods, to find tumors. The hybrid procedures involve the illumination of the breast tissues with microwaves, followed by their absorption [30].
-
-
- Microwave Tomography Technique
A developing biological imaging method called microwave tomography (MWT) uses the inverse scattering methodology to identify the tissue's dielectric properties. For non-invasive biological imaging, MWT is widely employed in medicine. The lowest and highest frequencies used in clinical MWT are 500 MHz and 30 GHz, respectively. The three primary parts of it are the picture reconstruction method, the interface, and the sensor system. It has a dielectric contrast as a result. It uses inversion scattering to create a permittivity and conductivity chart. The breast is completely encased by a cylinder-shaped antenna system that is lowered into it by a microwave tomography breast cancer inquiry device. One key benefit of MWT imaging is safety: unlike CT and nuclear medicine, which use ionizing radiation, MWT imaging uses a non-ionizing electromagnetic (EM) field [30,31].
-
-
- Radar-Based Technique
Imaging technology based on UWB (Ultra-Wideband) microwave radar uses reflected waves to produce images of breast tissue, frequently using a beamforming technique. During this procedure, a transmitting (TX) antenna on a radar system emits a UWB pulse that penetrates the breast tissue. Due to the varying dielectric characteristics at these tissue boundaries, some of the energy is reflected back when this pulse comes into contact with different tissue types, including fat, glandular tissue, and possible tumors [32].
The receiving (RX) components of the radar system then gather the reflected signals, which are mostly co-polarized reflections. The system can examine how the microwaves interacted with various materials in the breast thanks to these signals, which contain important information about the tissue structure. The system is able to reconstruct images that highlight areas of concern, like tumors, by processing these reflected signals through complex algorithms that take into account their unique dielectric signatures. This method improves the early detection and diagnosis of breast cancer by enabling detailed visualization of breast tissue [33].
-
- Breast Biopsy: Tissue Sampling
In a breast biopsy, a sample of breast tissue is taken for analysis, mainly to identify or rule out breast cancer or other anomalies. Usually, the procedure is carried out when a medical professional finds an abnormality during a physical examination or imaging test, like an ultrasound or mammography. There are various kinds of biopsies, such as core needle biopsy, which eliminate a greatest part of tissue; fine needle aspiration (FNA), that utilize a thin needle to eliminate fluid or tissue; and surgical biopsies, such as incisional or excisional biopsies, in which part or the entire abnormal area is surgically removed. To precisely target the area, imaging guidance may be utilized during the procedure, which frequently involves the administration of local anesthesia. Following collection, the tissue sample is examined by a pathologist to look for malignant growths. Biopsies are essential for diagnosis, but they are not screening tests; they do not lower the risk of dying from breast cancer, and they may result in false positives or negatives, which could cause needless worry or extra procedures. Overall, breast biopsies are essential for precisely determining the health of the breasts and directing future treatment choices [34].
-
- Liquid Biopsies: A Revolution in the Diagnosis of Cancer
Significant progress has been made in the identification and validation of novel biomarkers for breast cancer in recent years. Liquid biopsies, which analyze blood and other bodily fluids to find cellular or molecular alterations linked to breast cancer, are one well-known example. It is determined that this prospective alternative technique clearly outperforms traditional sampling biopsy, especially for patients with tumors that are physically challenging to sample directly [35].
The most developed of these new biomarkers,
-
-
- Circulating Tumor Cells (CTCs)
In tumor patients' peripheral blood, CTCs are dispersed among billions of erythrocytes and leukocytes [36]. The development of modern technology makes it possible to identify CTCs in biological fluids and enhances the sensitivity and accuracy of detection [37].
The quantity and existence of CTCs in a person's blood can currently only be tracked using two FDA-approved devices, Parsortix® and CELLSEARCH®. Parsortix® employs size-based, antigen-independent microfluidics technology, whereas CELLSEARCH® uses antigen-dependent immunomagnetic positive selection to collect CTCs based on EpCAM expression [38].
-
-
- Circulating Tumor DNA (ctDNA)
Small DNA fragments released into the bloodstream by tumor cells are known as circulating tumor DNA (ctDNA), and it has demonstrated great promise in a number of clinical contexts. It offers a sensitive way to use liquid biopsies, which are less invasive blood draws, to identify and track tumor-specific abnormalities [39].
By identifying cancer in its early stages, frequently before symptoms appear or conventional imaging techniques are efficient, it can help with early diagnosis. Additionally useful for tracking minimal residual disease (MRD) following treatment, ctDNA enables the early identification of possible relapses. Additionally, since alterations in ctDNA levels before and after treatment can reveal treatment efficacy, it can aid in predicting pathologic complete response (pCR) after neoadjuvant therapy. Furthermore, by locating particular mutations, ctDNA analysis can direct targeted therapy, allowing for treatment to be customized to the tumor profile of the individual. Lastly, it can identify mutations linked to treatment resistance, like PI3K or ESR1 mutations, which helps doctors make timely changes to their treatment plans. All things considered, ctDNA holds promise for greatly advancing cancer management through better early detection, monitoring, and individualized treatment strategies [40, 41].
-
- Signatures of Gene Expression: Tracking Genetic Traces of Tumors
Gene expression profiling was used to successfully classify breast cancer into molecular subtypes. These subtypes, which differ in their clinical features and responses to therapy, include luminal A and B, HER2-enriched, basal, and normal-like [42]. The use of multiparametric gene expression profiles (MammaPrint, Oncotype DX, Endo predict, and Prosigna/PAM50) as predictive tools for the stratification of early-stage HR-positive patients according to the chance of recurrence and the possible advantage of additional (chemo)therapy has been further developed and validated [43]. The most popular signatures, Oncotype DX and MammaPrint were first confirmed retroactively, for example, in the NSABP B14/B20, 131,132 SWOG-8814, 133 TransATAC134, or RASTER135 studies. Their prognostic and predictive value was later verified by several significant prospective studies. The only multigene test approved by the NCCN is Oncotype DX, which employs 21 genes [44].
Genome Analyses
Hereditary risk assessment for breast cancer heavily relies on genetic testing. Due to modifications in gene patent regulations and advancements in gene sequencing technology, the field of genetic testing has rapidly grown [45].
The following genetic assays as shown in Figure 4. It can be performed on a tumor sample that has previously undergone surgery or biopsy. For these examinations, most patients won't require a further biopsy or additional surgery.

Figure 4: Genetic Assays
- Oncotype Dx™: In cases of early-stage breast cancer, the 10-year probability of tumor recurrence is determined using a gene expression profiling test known as Oncotype DX (ODX). This test may be chosen for patients with breast cancer that is HER2-negative, ER-positive, or PR-positive and has not spread to the lymph nodes. It might also be a possibility in certain situations, such as those involving women who have undergone menopause, if the cancer has spread to one or three lymph nodes [46].
- MammaPrintTM: This test may be used for individuals over 50 with HER2-negative, ER-positive, or PR-positive breast cancer that has spread to three or fewer lymph nodes. This test uses information from 70 genes to determine the likelihood of recurrence for early-stage breast cancer [47].
- BluePrint® assay (BP): This 80-gene signature identifies the intrinsic molecular subtypes of early-stage breast cancers by analyzing specific gene expression profiles of signaling pathways causing different subtypes (basal, luminal, and HER2 types) [48].
- EndoPredict: Those who have undergone menopause and have ER-positive, HER2-negative breast cancer that has progressed to three or fewer lymph nodes may choose to get this test. This test estimates the likelihood that the cancer will return within ten years of diagnosis by utilizing data from twelve different genes [49].
- Prosigna (PAM50) TM: For those with ER-positive, HER2-negative breast cancer that has not progressed to the lymph nodes and who have gone through menopause, this test is an option. This test calculates the likelihood that a cancer will return within ten years of diagnosis based on data from fifty genes [50].
- Ki-67 index: A protein called Ki-67 is upregulated in cells before to mitosis. In a tumor, a high proportion of cells expressing the Ki-67 protein indicates that the cells are proliferating quickly. A measure of how rapidly tumour cells is proliferating is the Ki-67 index, often known as a proliferative index. When individuals with stage I or II breast cancer who have undergone menopause are unable to receive the genetic tests mentioned above, the Ki-67 index can be utilized to assist patients and their physicians in determining whether or not to provide chemotherapy and hormonal treatment [51].
- Immunohistochemistry (IHC)
In immunohistochemistry (IHC), the use of monoclonal and polyclonal antibodies is essential for determining the tissue distribution of pertinent antigens in both healthy and diseased individuals [52]. This test estimates the chance that cancer will return within ten years after diagnosis using the ER, PR, and HER2 status together with the Ki-67 index from a tumor sample. When the aforementioned genetic tests are unavailable, they can be utilized for patients whose cancer has either not progressed to any lymph nodes or has only affected one to three lymph nodes. The results of this test can assist patients and their physicians in deciding if hormone treatment should be administered before chemotherapy [53].
-
- Breast Cancer Index (BCI)
This test calculates the likelihood that the cancer will return five to ten years following a diagnosis based on data from eleven different genes. It's applied to patients whose cancer has either not spread to any lymph nodes or has only affected one to three. This test can assist patients and their physicians in determining if further hormonal therapy with tamoxifen, an AI, or tamoxifen followed by an AI is necessary for a patient who has had hormonal therapy for five years and shows no signs of cancer recurrence [54,55].
-
- Thermography
Thermography uses a specialized heat-sensing camera to measure the temperature of the skin covering the breasts. Although thermography has not yet been the focus of randomized clinical trials to ascertain its accuracy in detecting breast cancer or its risks, temperature variations caused by tumors may be visible on the thermogram [56].
-
- Artificial Intelligence: A New Approach to the Detection of Cancer
Over the past ten years, AI has raised the bar for human civilization in the creation of technologies that affect many different sectors to advance. Subfields of artificial intelligence (AI) technology include machine learning, deep learning, and computer vision. As AI is increasingly used in routine medical practice, there has been a noticeable advance in medical image and signal processing, medical resources management, medical workflow optimization, medical education, and other applications. AI clinical decision-making can help radiologists diagnose breast cancer through medical image processing, which will also enhance patient care. Because of advancements in medical imaging, radiologists' workflow has changed, and algorithms have the potential to enhance treatment beyond what is now possible for humans to accomplish. When it comes to image interpretation, AI may help radiologists recognize and categorize illness patterns in pictures and recommend the best course of treatment for a patient after consulting with other medical professionals engaged in the patient's care [57,58].
Advances in medical imaging have altered the workflow of radiologists, and algorithms have the potential to improve treatment beyond what is currently achievable by humans. When it comes to image interpretation, AI may help radiologists recognize and categorize illness patterns in pictures and recommend the best course of treatment for a patient after consulting with other medical professionals engaged in the patient's care. With its many uses, artificial intelligence (AI) is still revolutionizing many aspects of our lives. Results are considerably more easily and conveniently obtained when AI is used in the current screening procedure. Among the advantages of using AI techniques for breast cancer screening are faster and more precise findings [59].
-
- Deep Learning Techniques: Improving Screening Operations
Since artificial intelligence (AI) was introduced, deep learning techniques have been successfully used in breast cancer detection, which has improved patient survival rates by facilitating early diagnosis. A machine learning technique known as "deep learning" uses learning representation to automatically extract feature representations from incoming data [60]. Deep learning techniques include generative adversarial networks (GAN), multi-layer perceptrons, recurrent neural networks (RNN), deep autoencoders (AE), convolutional neural networks (CNN), and restricted Boltzmann machines (RBM) that have been proposed in recent decades [61-65] as shown in Figure 5.

Figure 5: Deep learning techniques
- Convolutional Neural Networks (CNN): CNN is an interconnected neural network with a hierarchical structure. One common deep-learning technique that creates convolutional operations on unprocessed data is the CNN method.
- Recurrent Neural Networks (RNN): Generative adversarial networks (GAN), convolutional neural networks (CNN), deep autoencoders (AE), recurrent neural networks (RNN), multi-layer perceptrons, and restricted Boltzmann machines (RBM) are examples of deep learning techniques [63].
- Autoencoder (AE): Utilizing encoder and decoder units, the autoencoder approach outputs a duplicate of the input data.
- Generative Adversarial Network (GAN): Its specific objective is to use training data to train a generative technique that infers the distribution of the target data. The discriminative model, which estimates the likelihood that a sample of data originates from actual training data rather than the output, is also used.
- Restricted Boltzmann Machines (RBM): Recursive Bayesian Machine Learning (RBM) techniques leverage blocks in an avaricious, layer-by-layer fashion for both network training and feature extraction.
- Multilayer Perceptron (MLP): Nonlinear activation functions and extra layers are used in the MLP approach for feed-forward neural networks.
- Strategies Focused on the Patient
The patient experience must be given top priority in early diagnosis innovations. Important things to think about are:
4.1. Knowledge and Perception
Raising awareness of the warning signs, risk factors, and importance of screening for breast cancer is essential. Women can be encouraged to take part in routine screenings and promptly seek medical advice through educational campaigns.
4.2. Mobile Health Technologies and Telemedicine
Women can find it simpler to make appointments, access educational materials, and get screening reminders when telemedicine and mobile health apps are used to improve patient engagement. These technologies can be especially helpful in underprivileged areas where access to medical care is scarce.
-
- Encouragement of Care
The emotional and psychological effects of receiving a breast cancer diagnosis can be lessened by incorporating supportive care services into diagnostic procedures. The general well-being of patients can be enhanced by offering resources for counseling and mental health support.
FUTURE DIRECTIONS AND DIFFICULTIES
Even with the encouraging developments in early diagnosis, a number of obstacles still exist [67-69]:
5.1. Expense and Accessibility
The high cost of new diagnostic technologies can prevent some populations from using them. To close the gap in healthcare, it is crucial to guarantee fair access to cutting-edge diagnostic equipment.
5.2. Regulatory Obstacles
Diagnostic method innovations must first pass stringent validation and regulatory approval before being widely used. It is essential to streamline this procedure while maintaining efficacy and safety.
-
- Clinical Practice Integration
Healthcare professionals must receive continual training and education in order to successfully incorporate new diagnostic tools into clinical workflows. The key to enhancing patient care is making sure that clinicians have the tools they need to use these innovations.
CONCLUSION
Rapid technological advancements and a better understanding of the disease are causing a radical transformation in the field of breast cancer diagnostics. Early detection is still crucial for effective treatment, as it significantly affects patient survival and quality of life. The incorporation of cutting-edge imaging technologies, including MRI, ultrasound, digital mammography, and 3D tomosynthesis, has greatly improved our capacity to identify breast cancer early on. Tumors that might not have been visible using conventional methods can now be better identified thanks to these techniques, which offer clearer and more detailed views of breast tissue. By evaluating metabolic activity, which is essential for comprehending tumor behavior and possible aggressiveness, modalities such as PET and PET/MRI provide an additional level of diagnostic power. The development of genetic testing and biomarker research is essential to tailoring breast cancer screening and treatment plans. Customizing breast cancer screening and treatment regimens requires the advancement of genetic testing and biomarker research. The way we interpret diagnostic data will be completely transformed by artificial intelligence. AI can greatly lower human error and produce more accurate diagnoses by improving image analysis and predictive modeling. As we continue to investigate these developments, changing the landscape of breast cancer diagnosis and treatment will require a multimodal strategy that tends to put patient-centered care and universal access first. Prioritizing early diagnosis through creative approaches can raise survival rates, improve quality of life, and eventually pave the way for a time when breast cancer is identified earlier and treated more successfully. We can ensure that breast cancer is identified earlier, treated more successfully, and eventually managed more successfully in the future by investing in healthcare infrastructure, education, and research.
ACKNOWLEDGEMENT
The infrastructure needed to complete the review work was provided by Smt. Kishoritai Bhoyar College of Pharmacy, Kamptee, for which the authors are grateful.
CONFLICT OF INTEREST
All authors have no conflicts of interest, whether they involve financial or non-financial resources.
REFERENCE
- H. Sung et al., "Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries," CA A Cancer J Clinicians, vol. 71, no. 3, pp. 209–249, May 2021. doi: 10.3322/caac.21660.
- E. I. Obeagu and G. U. Obeagu, "Breast cancer: A review of risk factors and diagnosis," Medicine, vol. 103, no. 3, Article e36905, Jan. 2024. doi: 10.1097/MD.0000000000036905.
- A. Bhushan, A. Gonsalves, and J. U. Menon, "Current State of Breast Cancer Diagnosis, Treatment, and Theranostics," Pharmaceutics, vol. 13, no. 5, Article 723, May 14, 2021. doi: 10.3390/pharmaceutics13050723.
- N. M. U. Din, R. A. Dar, M. Rasool, and A. Assad, "Breast cancer detection using deep learning: Datasets, methods, and challenges ahead," Computers in Biology and Medicine, vol. 149, Article 106073, Oct. 2022. doi: 10.1016/j.compbiomed.2022.106073.
- World Health Organization, Guide to cancer early diagnosis, Geneva, Switzerland: World Health Organization, 2017. Available: https://iris.who.int/handle/10665/254500.
- W. Ren, M. Chen, Y. Qiao, and F. Zhao, "Global guidelines for breast cancer screening: A systematic review," The Breast, vol. 64, pp. 85–99, Aug. 2022. doi: 10.1016/j.breast.2022.05.005.
- L. Nicosia et al., "History of Mammography: Analysis of Breast Imaging Diagnostic Achievements over the Last Century," Healthcare, vol. 11, no. 11, Article 1596, May 30, 2023. doi: 10.3390/healthcare11111596.
- R. Calandrino, A. Loria, P. Panizza, A. Taibi, and A. D. Vecchio, "State of art and optimization perspectives for breast imaging," Physics Open, vol. 7, Article 100071, May 2021. doi: 10.1016/j.physopen.2021.100071.
- L. Wilkinson, V. Thomas, and N. Sharma, "Microcalcification on mammography: Approaches to interpretation and biopsy," BJR, vol. 90, no. 1069, Article 20160594, Jan. 2017. doi: 10.1259/bjr.20160594.
- I. B. D. Paula and A. M. Campos, "Breast imaging in patients with nipple discharge," Radiol Bras, vol. 50, no. 6, pp. 383–388, Nov. 2017. doi: 10.1590/0100-3984.2017.50.6e10.
- K. Loizidou, R. Elia, and C. Pitris, "Computer-aided breast cancer detection and classification in mammography: A comprehensive review," Computers in Biology and Medicine, vol. 153, Article 106554, Feb. 2023. doi: 10.1016/j.compbiomed.2023.106554.
- S. Sharma and P. Khanna, "Computer-Aided Diagnosis of Malignant Mammograms using Zernike Moments and SVM," J Digit Imaging, vol. 28, no. 1, pp. 77–90, Feb. 2015. doi: 10.1007/s10278-014-9704-2.
- G. E. Park, B. J. Kang, S. H. Kim, and J. Lee, "Retrospective Review of Missed Cancer Detection and Its Mammography Findings with Artificial-Intelligence-Based, Computer-Aided Diagnosis," Diagnostics, vol. 12, no. 2, Article 387, Feb. 2, 2022. doi: 10.3390/diagnostics12020387.
- T. B. Bevers, "Ultrasound for the screening of breast cancer," Curr Oncol Rep, vol. 10, no. 6, pp. 527–528, Nov. 2008. doi: 10.1007/s11912-008-0057-5.
- A. Glechner et al., "Mammography in combination with breast ultrasonography versus mammography for breast cancer screening in women at average risk," Cochrane Database of Systematic Reviews, vol. 2023, no. 3, Mar. 31, 2023. doi: 10.1002/14651858.CD009632.pub3.
- Z. Zhang, "Detection of Breast Cancer with Addition of Annual Screening Ultrasound or a Single Screening MRI to Mammography in Women with Elevated Breast Cancer Risk," JAMA, vol. 307, no. 13, pp. 1394, Apr. 4, 2012. doi: 10.1001/jama.2012.391.
- M. L. Giger et al., "Automated Breast Ultrasound in Breast Cancer Screening of Women with Dense Breasts: Reader Study of Mammography-Negative and Mammography-Positive Cancers," American Journal of Roentgenology, vol. 206, no. 6, pp. 1341–1350, Jun. 2016. doi: 10.2214/AJR.15.15502.
- S. Radhakrishna et al., "Role of magnetic resonance imaging in breast cancer management," South Asian J Cancer, vol. 7, no. 2, pp. 69–71, Apr. 2018. doi: 10.4103/sajc.sajc_48_17.
- H. I. Greenwood et al., "Ductal Carcinoma in Situ of the Breasts: Review of MR Imaging Features," RadioGraphics, vol. 33, no. 6, pp. 1569–1588, Oct. 2013. doi: 10.1148/rg.336125708.
- S. Mennella et al., "Magnetic resonance imaging of breast cancer: Does the time interval between biopsy and MRI influence MRI–pathology discordance in lesion sizing?" Acta Radiol, vol. 58, no. 7, pp. 800–808, Jul. 2017. doi: 10.1177/0284185117693151.
- A. Janjic et al., "Gradient-Boosting Algorithm for Microwave Breast Lesion Classification—SAFE Clinical Investigation," Diagnostics, vol. 12, no. 12, Article 3151, Dec. 13, 2022. doi: 10.3390/diagnostics12123151.
- S. Kwon and S. Lee, "Recent Advances in Microwave Imaging for Breast Cancer Detection," International Journal of Biomedical Imaging, vol. 2016, Article 1–26, 2016. doi: 10.1155/2016/2984237.
- S. A. Taif, "Breast Magnetic Resonance Imaging Indications in Current Practice," Asian Pacific Journal of Cancer Prevention, vol. 15, no. 2, pp. 569–575, Jan. 30, 2014. doi: 10.7314/APJCP.2014.15.2.569.
- F. Kilic et al., "Diagnostic Magnetic Resonance Imaging of the Breast," EAJM, vol. 44, no. 2, pp. 106–114, Aug. 1, 2012. doi: 10.1111/j.2042-7346.2012.01247. x.
- J. Vercher-Conejero et al., "Positron Emission Tomography in Breast Cancer," Diagnostics, vol. 5, no. 1, pp. 61–83, Mar. 16, 2015. doi: 10.3390/diagnostics5010061.
- M. Shawky, Z. A. E. Ali, D. H. Hashem, and M. Houseni, "Role of positron-emission tomography/computed tomography (PET/CT) in breast cancer," Egypt J Radiol Nucl Med, vol. 51, no. 1, pp. 125, Dec. 2020. doi: 10.1186/s43055-020-00076-w.
- I. Iakovou et al., "Positron emission tomography in breast cancer: 18F-FDG and other radiopharmaceuticals," European J Hybrid Imaging, vol. 2, no. 1, Article 20, Dec. 2018. doi: 10.1186/s41824-018-0015-4.
- M. A. Aldhaeebi et al., "Review of Microwaves Techniques for Breast Cancer Detection," Sensors, vol. 20, no. 8, Article 2390, Apr. 22, 2020. doi: 10.3390/s20082390.
- A. A. Abdul Halim et al., "Existing and Emerging Breast Cancer Detection Technologies and Its Challenges: A Review," Applied Sciences, vol. 11, no. 22, Article 10753, Nov. 15, 2021. doi: 10.3390/app112210753.
- L. Wang, "Microwave Imaging and Sensing Techniques for Breast Cancer Detection," Micromachines, vol. 14, no. 7, Article 1462, Jul. 21, 2023. doi: 10.3390/mi14071462.
- N. Khalid et al., "Emerging paradigms in microwave imaging technology for biomedical applications: Unleashing the power of artificial intelligence," npj Imaging, vol. 2, no. 1, Article 13, Jun. 3, 2024. doi: 10.1038/s41534-024-00071-x.
- N. AlSawaftah et al., "Microwave Imaging for Early Breast Cancer Detection: Current State, Challenges, and Future Directions," J Imaging, vol. 8, no. 5, Article 123, Apr. 23, 2022. doi: 10.3390/jimaging8050123.
- F. E. Zerrad et al., "Novel measurement technique to detect breast tumor based on the smallest form factor of UWB patch antenna," Int J Microw Wireless Technol, vol. 15, no. 2, pp. 227–235, Mar. 2023. doi: 10.1017/S1759078723000066.
- C. D. Lehman, A. Y. Lee, and C. I. Lee, "Imaging Management of Palpable Breast Abnormalities," American Journal of Roentgenology, vol. 203, no. 5, pp. 1142–1153, Nov. 2014. doi: 10.2214/AJR.14.13187.
- J. Kitz, D. Goodale, C. Postenka, L. E. Lowes, and A. L. Allan, "EMT-independent detection of circulating tumor cells in human blood samples and pre-clinical mouse models of metastasis," Clin. Exp. Metastasis, vol. 38, no. 1, pp. 97–108, Feb. 2021. doi: 10.1007/s10585-021-10135-9.
- H. Kim and K. U. Park, "Clinical Circulating Tumor DNA Testing for Precision Oncology," Cancer Res. Treat., vol. 55, no. 2, pp. 351–366, Apr. 15, 2023. doi: 10.4143/crt.2022.921.
- M. Sant, A. Bernat-Peguera, E. Felip, and M. Margelí, "Role of ctDNA in Breast Cancer," Cancers, vol. 14, no. 2, p. 310, Jan. 9, 2022. doi: 10.3390/cancers14020310.
- A. Höller, B. D. Nguyen-Sträuli, H. Frauchiger-Heuer, and A. Ring, "Diagnostic and Prognostic Biomarkers of Luminal Breast Cancer: Where are We Now?" BCTT, vol. 15, pp. 525–540, Jul. 2023.
- A. Telekes and A. Horváth, "The Role of Cell-Free DNA in Cancer Treatment Decision Making," Cancers, vol. 14, no. 24, p. 6115, Dec. 12, 2022. doi: 10.3390/cancers14246115.
- M. Baksh et al., "Circulating Tumor DNA for Breast Cancer: Review of Active Clinical Trials," Cancer Treat. Res. Commun., vol. 32, p. 100609, 2022. doi: 10.1016/j.ctarc.2022.100609.
- A. Campos-Carrillo et al., "Circulating Tumor DNA as an Early Cancer Detection Tool," Pharmacol. Ther., vol. 207, p. 107458, Mar. 2020. doi: 10.1016/j.pharmthera.2019.107458.
- M. Bou Zerdan et al., "Genomic Assays in Node Positive Breast Cancer Patients: A Review," Front. Oncol., vol. 10, p. 609100, Feb. 16, 2021. doi: 10.3389/fonc.2020.609100.
- F. Cognetti et al., "Multigene Tests for Breast Cancer: The Physician’s Perspective," Oncotarget, vol. 12, no. 9, pp. 936–947, Apr. 27, 2021. doi: 10.18632/oncotarget.28211.
- S. Paik et al., "Gene Expression and Benefit of Chemotherapy in Women with Node-Negative, Estrogen Receptor–Positive Breast Cancer," J. Clin. Oncol., vol. 24, no. 23, pp. 3726–3734, Aug. 10, 2006. doi: 10.1200/JCO.2006.06.1007.
- J. A. Lynch, V. Venne, and B. Berse, "Genetic Tests to Identify Risk for Breast Cancer," Semin. Oncol. Nurs., vol. 31, no. 2, pp. 100–107, May 2015. doi: 10.1016/j.soncn.2015.02.002.
- S. Thibodeau and I. A. Voutsadakis, "Prediction of Oncotype Dx Recurrence Score Using Clinical Parameters: A Comparison of Available Tools and a Simple Predictor Based on Grade and Progesterone Receptor," Hematol. Oncol. Stem Cell Ther., vol. 12, no. 2, pp. 89–96, Jun. 2019. doi: 10.1016/j.hemonc.2019.01.001.
- J. C. Haan et al., "MammaPrint and BluePrint Comprehensively Capture the Cancer Hallmarks in Early?Stage Breast Cancer Patients," Genes Chromosomes Cancer, vol. 61, no. 3, pp. 148–160, Mar. 2022. doi: 10.1002/gcc.22907.
- M. M. Kuilman et al., "BluePrint Breast Cancer Molecular Subtyping Recognizes Single and Dual Subtype Tumors with Implications for Therapeutic Guidance," Breast Cancer Res. Treat., vol. 195, no. 3, pp. 263–274, Oct. 2022. doi: 10.1007/s10549-022-06564-3.
- K. Almstedt et al., "EndoPredict® in Early Hormone Receptor-Positive, HER2-Negative Breast Cancer," Breast Cancer Res. Treat., vol. 182, no. 1, pp. 137–146, Jul. 2020. doi: 10.1007/s10549-020-05778-7.
- T. Nielsen et al., "Analytical Validation of the PAM50-Based Prosigna Breast Cancer Prognostic Gene Signature Assay and nCounter Analysis System Using Formalin-Fixed Paraffin-Embedded Breast Tumor Specimens," BMC Cancer, vol. 14, no. 1, p. 177, Dec. 2014. doi: 10.1186/1471-2407-14-177.
- A. S. Nahed and S. M. Shaimaa, "Ki-67 as a prognostic marker according to breast cancer molecular subtype," Cancer Biol. Med., vol. 13, no. 4, pp. 496, 2016.
- J. Duraiyan, R. Govindarajan, K. Kaliyappan, and M. Palanisamy, "Applications of immunohistochemistry," J. Pharm. Bioall. Sci., vol. 4, no. 6, pp. 307, 2012. doi: 10.4103/0975-7406.104796.
- D. C. Zaha, "Significance of immunohistochemistry in breast cancer," World J. Clin. Oncol., vol. 5, no. 3, pp. 382, 2014. doi: 10.5306/wjco.v5.i3.382.
- J. M. Sgroi, D. C. Bartlett, K. Treuner, Y. Zhang, I. Ahmed, T. Piper, et al., "Breast Cancer Index and prediction of benefit from extended endocrine therapy in breast cancer patients treated in the Adjuvant Tamoxifen—To Offer More? (aTTom) trial," Ann. Oncol., vol. 30, no. 11, pp. 1776–1783, Nov. 2019. doi: 10.1093/annonc/mdz285.
- J. M. Bartlett, D. C. Sgroi, K. Treuner, Y. Zhang, T. Piper, R. C. Salunga, et al., "Breast Cancer Index is a predictive biomarker of treatment benefit and outcome from extended tamoxifen therapy: Final analysis of the Trans-aTTom study," Clin. Cancer Res., vol. 28, no. 9, pp. 1871–1880, May 2, 2022. doi: 10.1158/1078-0432.CCR-21-3667.
- M. B. Rakhunde, S. Gotarkar, and S. G. Choudhari, "Thermography as a Breast Cancer Screening Technique: A Review Article," Cureus, Nov. 8, 2022. [Online]. Available: https://www.cureus.com/articles/113483-thermography-as-a-breast-cancer-screening-technique-a-review-article.
- G. Dileep and S. G. Gianchandani Gyani, "Artificial Intelligence in Breast Cancer Screening and Diagnosis," Cureus, Oct. 15, 2022. [Online]. Available: https://www.cureus.com/articles/106594-artificial-intelligence-in-breast-cancer-screening-and-diagnosis.
- J. S. Ahn, S. Shin, S. A. Yang, E. K. Park, K. H. Kim, S. I. Cho, et al., "Artificial intelligence in breast cancer diagnosis and personalized medicine," J. Breast Cancer, vol. 26, no. 5, pp. 405, 2023. doi: 10.4048/jbc.2023.26.e45.
- K. Dembrower, A. Crippa, E. Colón, M. Eklund, and F. Strand, "Artificial intelligence for breast cancer detection in screening mammography in Sweden: A prospective, population-based, paired-reader, non-inferiority study," Lancet Digit. Health, vol. 5, no. 10, pp. e703–e711, Oct. 2023. doi: 10.1016/S2589-7500(23)00194-X.
- M. Nasser and U. K. Yusof, "Deep learning based methods for breast cancer diagnosis: A systematic review and future direction," Diagnostics, vol. 13, no. 1, pp. 161, Jan. 3, 2023. doi: 10.3390/diagnostics13010161.
- I. H. Sarker, "Deep learning: A comprehensive overview on techniques, taxonomy, applications and research directions," SN Comput. Sci., vol. 2, no. 6, pp. 420, Nov. 2021. doi: 10.1007/s42979-021-00656-w.
- V. Nemade, S. Pathak, and A. K. Dubey, "A systematic literature review of breast cancer diagnosis using machine intelligence techniques," Arch. Comput. Methods Eng., vol. 29, no. 6, pp. 4401–4430, Oct. 2022. doi: 10.1007/s11831-021-09675-0.
- H. Yao, X. Zhang, X. Zhou, and S. Liu, "Parallel structure deep neural network using CNN and RNN with an attention mechanism for breast cancer histology image classification," Cancers, vol. 11, no. 12, pp. 1901, Nov. 29, 2019. doi: 10.3390/cancers11121901.
- N. Simidjievski, C. Bodnar, I. Tariq, P. Scherer, H. A. Terre, Z. Shams, et al., "Variational autoencoders for cancer data integration: Design principles and computational practice," Front. Genet., vol. 10, pp. 1205, Dec. 11, 2019. doi: 10.3389/fgene.2019.01205.
- A. Swiecicki, N. Konz, M. Buda, and M. A. Mazurowski, "A generative adversarial network-based abnormality detection using only normal images for model training with application to digital breast tomosynthesis," Sci. Rep., vol. 11, no. 1, pp. 10276, May 13, 2021. doi: 10.1038/s41598-021-89744-9.
- R. Miotto, F. Wang, S. Wang, X. Jiang, and J. T. Dudley, "Deep learning for healthcare: review, opportunities and challenges," Brief. Bioinform., vol. 19, no. 6, pp. 1236–1246, Nov. 27, 2018. doi: 10.1093/bib/bbx044.
- J. Hamer and E. Warner, "Lifestyle modifications for patients with breast cancer to improve prognosis and optimize overall health," CMAJ, vol. 189, no. 7, pp. E268–E274, Feb. 21, 2017. doi: 10.1503/cmaj.160903.
- C. H. Barrios, "Global challenges in breast cancer detection and treatment," The Breast, vol. 62, pp. S3–S6, Mar. 2022. doi: 10.1016/j.breast.2022.01.011.


 Krishna Gupta*
Krishna Gupta*
 Kalyani Thombre
Kalyani Thombre
 Milind Umekar
Milind Umekar





 10.5281/zenodo.14186018
10.5281/zenodo.14186018