Abstract
The use of herbal medicines has grown in importance on a global scale, both medically and economically. Although the quality, safety, and efficacy of many natural medicines have improved with use. The oldest known type of healthcare is provided through herbal remedies. The creation and assessment of herbal gels were the goals of the current study project. Compared to other gels, herbal gels provide a number of benefits. Gels are semi-solid preparations with liquid-interpenetrating big organic molecules or tiny inorganic molecules. Using carbopol940, gel formulations were produced that had good homogeneity, no skin irritation, good stability, and anti- inflammatory efficacy. Gels made with herbal ingredients are preferable to those made with synthetic ingredients since they have fewer adverse effects. For their effective antibacterial, antiseptic, antioxidant, and anti-inflammatory properties, these herbal plants have been used for a very long time to keep skin healthy, clear, andshiny while fending off numerous challenges including dryness, roughness, skin redness, and acne. The procedures used to make herbal gel were fairly straight forward. In this experiment, Punica granatum was utilized to prepare a herbal gel. Pomegranate fruits must be manually chopped to separate the arils and peel after being rinsed with distilled water. Pomegranate juice must be physically squeezed out of the arils. Pomegranate juice is placed in a beaker and heated for a period of time at 40 o C. After that, the necessary amount of Carbopol 940 is added while stirring continuously. By boiling it in a water bath, the necessary amount of tocopherol was added. This mixture is now properly combined, and it should be stored in an airtight container. The herbal gel made above was assessed. To examine the performance of this composition, more research is required.
Keywords
Herbal gel, Punucagranatum, antioxidant, anti-inflammatory activity
Introduction
Herbal Gel- Gels are semi-solid preparations with liquid-interpenetrating big organic molecules or tiny inorganic molecules[1]. Xanthan gum, carbopol940, and other ingredients in the gel formulation demonstrated good homogeneity, no skin irritation, good stability, and anti-inflammatory efficacy[2]. Gels made with herbal ingredients are preferable to those made with synthetic ingredients since they have fewer adverse effects[1,2]. These medicinal plants have been used for a very long time because of their potent antibacterial, antiseptic, antioxidant, and anti-inflammatory properties, which help to keep skin healthy, clear, and shiny in the face of diverse challenges like dryness, roughness, skin redness, and acne. For various formulations, different fruits can be utilized, such as apples, papayas, strawberries, watermelons, etc[3].
Advantages of herbal gels
They are quickly absorbed in to the skin.
They do not cause allergic reactions or adverse side effects.
Skin- and Earth-friendly[1,3].
Types of gels
Single phase and two phase, based on the number.
Based on whether the gelling ingredientis natural or artificial.
Organic and inorganic gelling agents,depending on their composition.
Hydrophilic and hydrophobic solvents, based on the type of solvent.
The word "cosmetic" comes from the Greek word "cosmetics," which denotes a cosmetic or material that enhances beauty. There are two purposes for these cosmetics:
The up holding, restoring,or conferring of physical attractiveness.
Surgically repairing a physically deformity[4].
Cosmeceuticals are a combination of cosmetics and medications, commonly including products like anti-aging creams and moisturizers. In Ayurveda, cosmetics are referred to as Varnya or Twakdohhargunas. Recently, the Ministry of AYUSH in India recognized the "Saundarya Poshak" category under the Drugs and Cosmetics Act of 1940 and its associated rules. Modern cosmetic products widely used today include creams, lotions, gels, oils, soaps, shampoos, and hair dyes[5].
Fruits are rich in antioxidants and vitamins, offering numerous health benefits. The pomegranate (Punica granatum L.) from the Punicaceae family is especially valued for its high antioxidant content. Native to Southern Asia, it is cultivated in countries like India, Lebanon, Spain, China, and the USA, among others[6]. The pomegranate fruit is spherical, with a uniform skin tone and divided into chambers containing seeds surrounded by juicy arils. Its juice is popular for consumption and is also used in culinary applications like making jellies. The pomegranate tree is a small, thorny tree growing 5–10 meters tall, with a lifespan of up to 200 years in some cases. Its leaves are narrow, shiny, and 3–7 cm long, while its vibrant red flowers have 3–7 petals. Certain cultivars of pomegranate exist that do not produce fruit. The pomegranate (Punica granatum L.) is a fruit-bearing tree of the Punicaceae family, widely recognized for its medicinal, nutritional, and cultural significance. The fruit comprises an outer hard shell (pericarp) and an inner reddish-purple mesocarp with asymmetrically arranged membranes forming chambers. These chambers house seeds encased in fleshy seed coats called sarcotestas, which are juicy and nutrient-rich. Each fruit contains 200–1,400 seeds and measures 5–12 cm in diameter[7]. A symbol of fertility and longevity, the pomegranate has antioxidant, anti-inflammatory, and anti-parasitic properties due to bioactive compounds such as anthocyanins, flavonoids, phenolic acids, alkaloids, and tannins. Pomegranate seed oil, rich in punicic acid, offers potential benefits for diabetes and cancer prevention. With over 500 varieties worldwide, pomegranates are consumed fresh or processed into juices, jams, syrups, wines, and other products[8]. Juice from the whole fruit has higher polyphenol and antioxidant content compared to juice from arils alone, attributed to the migration of phenolic compounds during processing. The fruit’s bioactive components, including phenolic acids, flavonoids, and hydrolysable tannins, contribute to its health benefits, such as reducing the risk of cardiovascular disease, cancer, diabetes, and neurological disorders[9]. Pomegranate juice's antioxidant activity surpasses red wine and green tea, while its phytochemistry supports applications in preventing and treating ailments like atherosclerosis, dental issues, and skin damage. The fruit's rising popularity has spurred global cultivation and research, emphasizing its multifaceted uses in nutrition, medicine, and industry. Pomegranate seed oil is effective for skin health due to flavonoids and punicic acid, which suppress pro-inflammatory enzymes and promote keratinocyte division, aiding epidermal thickening. It can prevent certain skin cancers at 5% concentration and reduce wrinkles via phyto estrogens. Pomegranate juice, rich in polyphenols (280–560 mg/L), supports cardiovascular health, reduces oxidative stress, and complements established therapies, although it isn't a standalone treatment[8,9]. Ellagic acid and flavonoids like quercetin in pomegranate juice exhibit strong antioxidant and anticancer effects. Ellagic acid absorption is evidenced by increased serum levels post-consumption, with metabolites detectable for up to 48 hours. Pomegranate polyphenols inhibit inflammatory proteins (e.g., COX-2), combat bacterial and viral infections, and show potential against cancers, including breast, colon, and prostate. Punicalagin also reduces fat production by 40% and improves vascular function in metabolic syndrome and atherosclerosis[10]. Antioxidant-rich pomegranate peel (PP) contains polyphenols, tannins, and flavonoids, which counter oxidative stress and inflammation, reducing risks for diseases like cancer and atherosclerosis. Its components, including punicalin, punicalagin, ellagic acid, and flavonoids (e.g., quercetin), have medicinal and cosmetic applications. Peel extracts display higher phenolic content than arils, with optimal harvesting times linked to peak nutritional value. PP supplementation boosts antioxidant enzymes like PON1 and PON2 while reducing oxidative stress[9,10]. Studies highlight pomegranate's antioxidant, anti-inflammatory, and anticancer properties, supported by its bioactive compounds. Its peel and juice show diverse applications in traditional medicine, cosmetics, and health supplements. Pomegranate peels and extracts are rich in natural antioxidants like tannins, flavonoids, and phenolics, which combat oxidative stress, enhance food stability, and provide health benefits. They have antiviral, antifungal, and antimicrobial properties, making them valuable in traditional medicine for conditions like infections, inflammation, and cancer prevention. With rising concerns over synthetic antioxidants, pomegranate peels offer a sustainable, effective alternative for use in food preservation, supplements, and cosmetics.
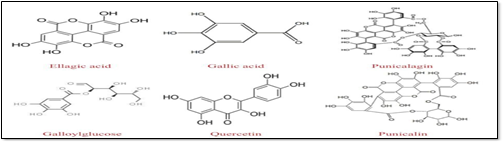
Fig1:Structure of chemical constituents present in Punica
Pomegranate seeds, rich in phenolics, antioxidants, and bioactive compounds, have long been valued in traditional medicine and as a source of health-promoting oil with anti-carcinogenic properties. Advanced extraction methods like ultrasonic-assisted extraction enhance yield and preserve antioxidant activity. Herbal remedies, including gels made with simple processes, are increasingly significant globally for their therapeutic benefits and ease of preparation[12].
Antioxidants:- Antioxidants prevent oxidation, protect against free radicals, and are used in foods to prevent spoilage and in industries to avoid material degradation. Natural antioxidants like glutathione and vitamins A, C, and E safeguard cells, though the health effects of other labeled substances remain unclear. Plants evolved antioxidants millions of years ago as defenses against reactive oxygen species, and the discovery of vitamins C and E highlighted their essential role in preventing oxidative damage[13].
Synthetic and Natural Antioxidants:- Synthetic antioxidants are used in medicine, food, and industry, with some mimicking natural antioxidant chemistry and others offering selective protection without structural similarity. They stabilize materials like plastics and rubber but require careful consideration of toxicological impacts when used in food. Natural antioxidants from plants, particularly phenolics, are explored for preserving meat products. Flavonoids, a class of polyphenols, are well-studied for their antioxidant properties[14].
Mechanism of Action:- Antioxidants: Scavenge free radicals (e.g., flavonoids, phenolic acids). Inhibit metal-catalyzed oxidation by forming complexes. Decompose peroxides to stable compounds (e.g., glutathione peroxidase). Inactivate singlet oxygen. Block enzymatic pathways for auto-oxidation.
Health Hazards- While antioxidants protect against free-radical damage, synthetic variants carry potential risks, highlighting the need for safe natural alternatives.
Pomegranate Antioxidants- Pomegranate (Punica granatum) is rich in bioactive compounds and has potent antioxidant properties, especially in its peel. Studies show the peel outperforms the pulp in antioxidant capacity due to higher phenolic and flavonoid content, offering greater health benefits[15].
Antimicrobial and Anti-inflammatory Activity of Pomegranate:- Pomegranate has long been used in traditional medicine for its antimicrobial properties, showing effectiveness against bacteria like Staphylococcus epidermidis and Klebsiella pneumoniae. Its antibacterial activity varies based on the concentration of phenolic compounds, pigments, and citric acid. Pomegranate seeds and extracts have demonstrated activity against pathogens like Bacillus subtilis, Escherichia coli, and Saccharomyces cerevisiae. The anti-inflammatory effects of pomegranate stem from compounds like ellagitannins, ellagic acid, flavonoids, and tannins found in the peel, juice, and other parts. These constituents inhibit inflammation through various mechanisms, including reducing oxidative stress and blocking inflammatory pathways[16].
Medicinal Uses of Pomegranate:- Historically, pomegranate has been used across cultures for ailments ranging from intestinal worms to wounds, diarrhea, and infections. Ancient texts and traditions from Egypt, Greece, Persia, and China highlight its significance in medicine and symbolism. The fruit’s bark, rind, flowers, and seeds have been utilized for their therapeutic properties, including astringent, hemostatic, and antimicrobial effects. Rich in bioactive compounds like tannins, phenolics, and estrone, pomegranate aids in treating infections, inflammation, and other conditions like atherosclerosis, skin injuries, and food poisoning. Its natural antimicrobial agents offer potential for food preservation and disease management, though associated flavors can impact food taste. Pomegranate’s diverse medicinal properties make it a valuable source for developing natural antimicrobial and therapeutic agents, especially in combating microbial diseases and enhancing food stability[15,16].
Chemical constituents of pomegranate
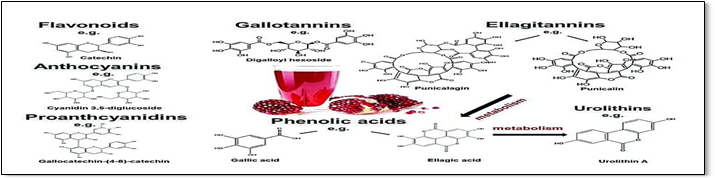
Fig2: chemical constituents of promgranate
Catechin
Catechin [(2R,3S)-2-(3,4-Dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol] is a flavan-3-ol with notable antioxidant properties.
- Physical Properties:
- Colorless solid
- Molar mass: 290.27 g/mol
- Melting point: 175–177°C
- Chemical Properties:
- Formula: C15H14O6
- Neutralizes reactive oxygen and nitrogen species
Highly soluble in alcohols, acetone, and polar organic solvents[17].
Gallotannin
Gallotannins, also known as tannic acid or gallotannic acid, belong to the hydrolyzable tannins.
- Physical Properties:
- Molecular weight: 1701.1 g/mol
- Light yellow to tan solid with a faint odor and astringent taste
- Boiling point: 218°C; Melting point: 200°C
- Chemical Properties:
- Formula: C76H52O46
- Hydrogen bond donor: 25; Acceptor: 46
- Soluble in water, glycerol, ethanol, and acetone[18].
Punicalagin
Punicalagin is an ellagitannin found in pomegranates, existing as alpha and beta isomers.
- Physical Properties:
- Molecular weight: 1084.72 g/mol
- Yellow-green powder
- Melting point: 155°C
- Chemical Properties:
- Formula: C48H28O30
- Soluble in water, methanol, ethanol, and organic solvents[19].
Punicalin
Punicalin is another ellagitannin derived from Punica granatum.
- Physical Properties:
- Molecular weight: 782.5 g/mol
- Dark yellow in color
- Chemical Properties:
- Formula: C34H22O22
- Hydrogen bond donor: 13; Acceptor: 22
- Soluble in water, methanol, and ethanol[20].
Proanthocyanidins
Proanthocyanidins are polyphenols with limited water solubility and weak acidity.
- Physical Properties:
- Molecular weight: 592.55 g/mol
- Melting point: 128–130°C; Boiling point: 368°C
- Chemical Properties:
- Formula: C31H28O12
- Hydrogen bond donor: 9; Acceptor: 12
GallicAcid
Gallic acid (3,4,5-trihydroxybenzoic acid) is a prominent phenolic acid in plants.
- Physical Properties:
- Molecular weight: 170.12 g/mol
- White to pale fawn crystals
- Melting point: 251°C; Boiling point: 259.73°C
- Chemical Properties:
- Formula: C7H6O5
- Soluble in alcohol and ether, but low solubility in water [21].
EllagicAcid
Ellagic acid, a polyphenol and dilactone of hexahydroxydiphenic acid, is present in Punica granatum.
- Physical Properties:
- Molecular weight: 302.19 g/mol
- Cream-colored solid, crystalline or powdery
- Chemical Properties:
- Formula: C14H6O8
- Soluble in organic solvents, with poor water solubility [22].
Carbopol
Carbopol polymers, cross-linked high molecular weight acrylic acid polymers, are anionic and exhibit excellent water absorption and swelling properties.
- Physical Properties:
- Appears as white/off-white powder
- Swells up to 1000 times its original volume in water and forms a gel in pH 4.0–6.0 environments
- Glass transition temperature: 105°C (221°F)
- Chemical Properties:
- Composed of acrylic acid cross-linked with allyl ethers of pentaerythritol
- Dissolves in water, alcohol, and glycerin when neutralized with alkali hydroxides or amines
- Viscosity (0.2% solution): 19,000–35,000 cPs [23].
Materials and instruments:- Extracts
- Pomegranate juice
- Ethanolic extract of pomegranate peel
- Ethanolic extract of pomegranate seed
Chemicals:
|
Distilled water
|
Prepared in lab
|
|
Ethanol
|
Chang shuhong shen fine chemical.,ltd
|
|
Tocopherol
|
Well pace nutrition
|
|
Carbopol940
|
Himedia laboratories pvt. ltd, mumbai
|
|
Leadacetate
|
Merck life science private limited, mumbai.
|
|
Hydrochloricacid
|
Merck life science private limited, mumbai.
|
|
Sulphuric acid
|
Merck life science private limited, mumbai.
|
|
Sodium hydroxide
|
Hi media laboratories pvt. ltd., mumbai
|
|
Ferric chloride
|
Siscore search laboratories pvt.ltd. ,maharashtra
|
|
Gelatin
|
Spectrumreagentandchemicalspvt.ltd
|
|
Sodium chloride
|
Emplura, mumbai
|
Instruments:
|
UVSpectrophotometer
|
Shimadzu,UV-1800,Malaysia
|
|
FTIR
|
Bruker
|
|
Deepfreezer
|
Elanpro
|
|
Microwave oven
|
Samsung
|
|
Hot plate
|
Relitech
|
|
Magnetic stirer
|
Remi
|
|
pH meter
|
Alpha-60
|
|
Double distillation unit
|
Borosil
|
|
Digital balance
|
Keroy,Varanasi
|
|
Varivol II Micropipette
|
HiMedia
|
Glass ware apparatus:
|
Beaker
|
Borosil
|
|
Conical flask
|
Borosil
|
|
Round bottom flask
|
Borosil
|
|
Pipette
|
Borosil
|
|
Glass rod
|
Borosil
|
|
Funnel
|
Borosil
|
|
Measuringcylinder
|
Borosil
|
|
Reagentbottol
|
Borosil
|
|
Testtube
|
Borosil
|
|
Petridish
|
Borosil
|
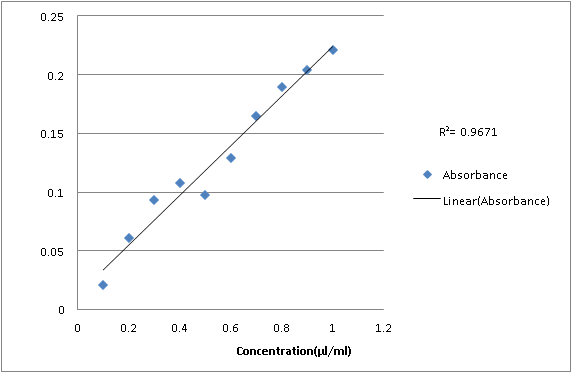
UV analysis of the ethanolic extract of pomegranate seed:-
First dilution:
|
Concentration(µl/ml)
|
Buffer
|
Stock
|
Absorbance
|
|
0.1
|
9
|
1
|
0.021
|
|
0.2
|
8
|
2
|
0.061
|
|
0.3
|
7
|
3
|
0.093
|
|
0.4
|
6
|
4
|
0.108
|
|
0.5
|
5
|
5
|
0.097
|
|
0.6
|
4
|
6
|
0.129
|
|
0.7
|
3
|
7
|
0.165
|
|
0.8
|
2
|
8
|
0.189
|
|
0.9
|
1
|
9
|
0.204
|
|
1
|
0
|
10
|
0.221
|
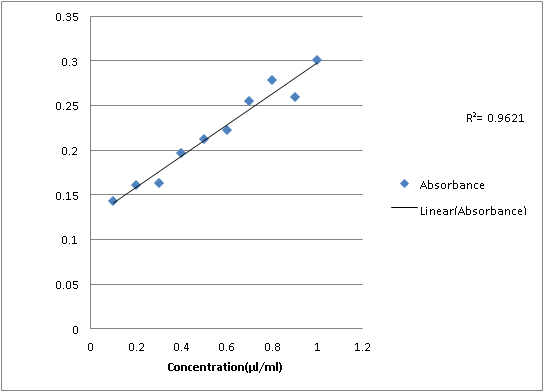
Second dilution:
|
Concentration(µl/ml)
|
Buffer
|
Stock
|
Absorbance
|
|
0.1
|
9
|
1
|
0.143
|
|
0.2
|
8
|
2
|
0.161
|
|
0.3
|
7
|
3
|
0.163
|
|
0.4
|
6
|
4
|
0.197
|
|
0.5
|
5
|
5
|
0.212
|
|
0.6
|
4
|
6
|
0.223
|
|
0.7
|
3
|
7
|
0.255
|
|
0.8
|
2
|
8
|
0.279
|
|
0.9
|
1
|
9
|
0.260
|
|
1
|
0
|
10
|
0.301
|
Third dilution:
|
Concentration(µl/ml)
|
Buffer
|
Stock
|
Absorbance
|
|
0.1
|
9
|
1
|
0.080
|
|
0.2
|
8
|
2
|
0.106
|
|
0.3
|
7
|
3
|
0.106
|
|
0.4
|
6
|
4
|
0.146
|
|
0.5
|
5
|
5
|
0.151
|
|
0.6
|
4
|
6
|
0.153
|
|
0.7
|
3
|
7
|
0.195
|
|
0.8
|
2
|
8
|
0.215
|
|
0.9
|
1
|
9
|
0.198
|
|
1
|
0
|
10
|
0.238
|
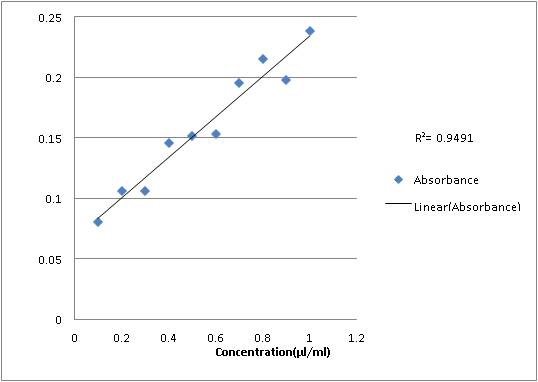
Fourth dilution:
|
Concentration(µl/ml)
|
Buffer
|
Stock
|
Absorbance
|
|
0.1
|
9
|
1
|
0.043
|
|
0.2
|
8
|
2
|
0.085
|
|
0.3
|
7
|
3
|
0.115
|
|
0.4
|
6
|
4
|
0.132
|
|
0.5
|
5
|
5
|
0.121
|
|
0.6
|
4
|
6
|
0.151
|
|
0.7
|
3
|
7
|
0.186
|
|
0.8
|
2
|
8
|
0.221
|
|
0.9
|
1
|
9
|
0.236
|
|
1
|
0
|
10
|
0.254
|
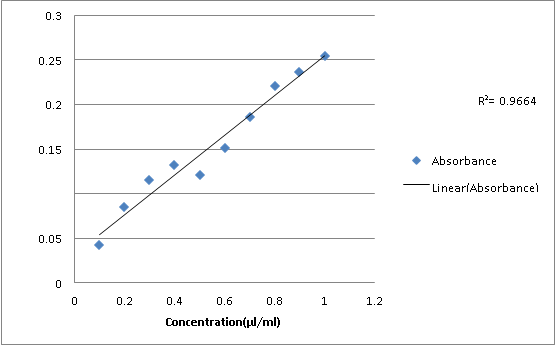
Fifth dilution:
|
Concentration(µl/ml)
|
Buffer
|
Stock
|
Absorbance
|
|
0.1
|
9
|
1
|
0.118
|
|
0.2
|
8
|
2
|
0.143
|
|
0.3
|
7
|
3
|
0.141
|
|
0.4
|
6
|
4
|
0.175
|
|
0.5
|
5
|
5
|
0.197
|
|
0.6
|
4
|
6
|
0.208
|
|
0.7
|
3
|
7
|
0.240
|
|
0.8
|
2
|
8
|
0.265
|
|
0.9
|
1
|
9
|
0.248
|
|
1
|
0
|
10
|
0.288
|
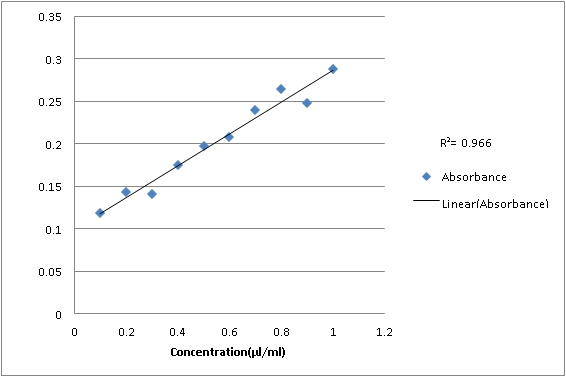
Sixth dilution:
|
Concentration(µl/ml)
|
Buffer
|
Stock
|
Absorbance
|
|
0.1
|
9
|
1
|
0.123
|
|
0.2
|
8
|
2
|
0.151
|
|
0.3
|
7
|
3
|
0.149
|
|
0.4
|
6
|
4
|
0.179
|
|
0.5
|
5
|
5
|
0.184
|
|
0.6
|
4
|
6
|
0.190
|
|
0.7
|
3
|
7
|
0.228
|
|
0.8
|
2
|
8
|
0.248
|
|
0.9
|
1
|
9
|
0.233
|
|
1
|
0
|
10
|
0.274
|

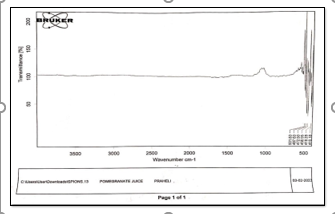
Figure 3:IR of Pomegranate juice
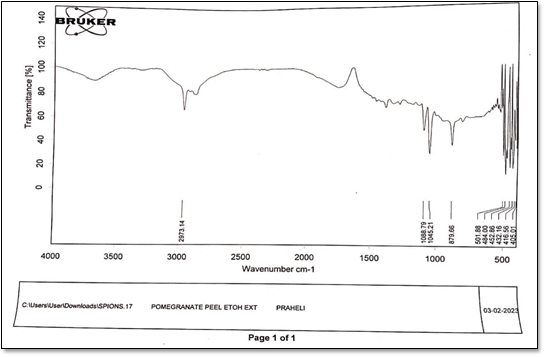
Figure4: IR of Pomegranate peel ethanolic extract
Figure5: IR of Pomegranate peel water extract
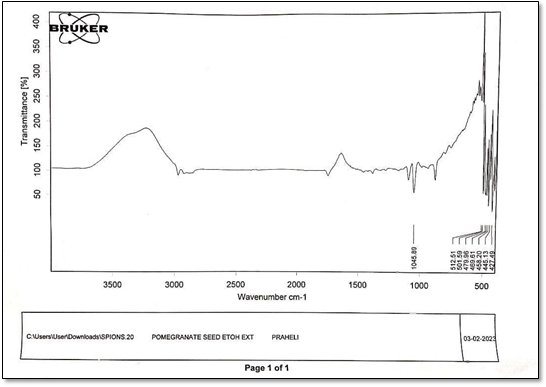
Figure6: IR of Pomegranate seed ethanolic extract
Probable peaks for performing IR spectroscopy:
For PCN, isolated from the husk of pomegranate extract, the spectrum was scanned over a wave number range of 4000–500 cm-1. At higher wave numbers, the FTIR spectrum of the PCS exhibited broadband at 3336 cm-1 corresponded to the strong intra molecular hydrogenbond (OH).Two quietly broad bandswere observed at 1738 and 655 cm-1 which have the stretching vibrations of the d(C = N), v(C=C=C), and m(C–H). The broad peak at 3300 cm-1 and 1643 cm-1 indicated the change in O–H and N–H stretching in PCN and indicated that the OH group was also involved in hydrogen bonding. The disappearance of bands in PCN at 1738 cm-1 indicated the breaking of C=C=C bonds. The changes in the FTIR confirm the similarity between PCS and PCN synthesized from pomegranate husk extract[24].
The FTIR spectrum of seed extractpeaks appear at 3449, 2073, 1400, 1638, 1053,675 cm-1 respectively. The band at 3449 cm-1 indicates phenolic OH. The band at 2073 cm-1 is due to stretching vibration of alkynes. The band at 1638 cm-1 is due to N-H bend of 1o amines. The band at 1400 is corresponds to C-C stretching vibration to aromatics. The band at 1053 cm-1 is due to C-N stretching vibration to aromatic amines. The band at 675 cm-1 is due to C-H bending of alkynes[25].
The FTIR spectrumofpeelextract peaks appear at 3364, 2931, 1639, 1090, 1639 cm- 1 respectively. The band at 3364 cm-1 is due to phenolic OH. The band 2931 cm-1 CH plane bends to alkenes. The band at 1639 cm-1 corresponds to N-H bends to 1oamines.Thebandat 694cm-1 isdueto C-Hbendto alkynes.Thebandcorresponds to 1090 cm-1 corresponds to C-H Wag to alkyl halides[26].
Observation and Evaluation:
Table1:Organoleptic Characters
|
S.NO
|
Name of the fruit parts
|
Nature
|
Color
|
Odor
|
Taste
|
|
1.
|
Pomegranate juice
|
Liquid
|
Red-purple
|
Sweet- fruity
|
Sweet-tart
|
|
2.
|
Pomegranate peel
|
Leathery skin
|
Redish brown color
|
Faint aromatic odor
|
Astringent test
|
|
3.
|
Pomegranate seed
|
Crunchy embryo
|
Yellowish white
|
Noodor
|
Bitter
|
Table2:Powder Microscopy
|
S.No
|
Fruit parts
|
Observation
|
|
1.
|
Pomegranate peel powder
|
Presence of different types of xylemvessels,stone cells and prismatic calcium oxalate crystal.
|
|
2.
|
Pomegranate seed powder
|
Presence of fiber,ashcontantsetc.
|
Table3: Tests for Pomegranate juice
|
Test
|
Perform
|
Observation
|
Decision
|
|
Alkaline reagent test
|
Tests olution+few drops of NaOH solution
|
Intense yellow colour is obtained which turns to colourless on addition of few dropsofdilutedacid
|
Flavonoids are present
|
|
Lead acetatetest
|
Testsolution(alcoholicextract ofguavaleave)+Leadacetate solution
|
Yellowprecipitation
|
Quercetinand other Flavonoidsare
present
|
|
Sulfuric acid test
|
Testsolution+sulfuricacid (66-80%)
|
Orangetoredcolor observed
|
Flavanones are present
|
|
Ferric chloride test
|
Test solution+ferricchloride solution (5%)
|
Brownishblueor green colour
|
Hydrolysable tannins are present
|
|
Gelatintest
|
Testsolution+1% gelatin
solution+10%NaCl solution
|
White buff
Colouredppt. formed
|
Tannin present
|
Table4: Tests for Pomegranate Peel ethanolic extract
|
Test
|
Perform
|
Observation
|
Decision
|
|
Alkaline reagent test
|
Test solution+fewdropsof NaOH solution
|
Intense yellow colour is obtained which turns to colourless on addition of few dropsofdilutedacid
|
Flavonoids are present
|
|
Lead acetatetest
|
Testsolution(alcoholicextract ofguavaleave)+Leadacetate solution
|
Yellow precipitation
|
Quercetinand other Flavonoidsare
present
|
|
Sulfuric acid test
|
Testsolution+sulfuric acid (66-80%)
|
Redtoredbluish color
|
Chalcones andaurones
present
|
|
Ferric chloride test
|
Testsolution+ferricchloride solution (5%)
|
Brownishblueor green color
|
Hydrolysable tannins are
present
|
|
Gelatin test
|
Testsolution+1% gelatin
solution+10%NaCl solution
|
Whitebuffcolored ppt. formed
|
Tannins present
|
Table5:Tests for Pomegranate Peel water extract
|
Test
|
Perform
|
Observation
|
Decision
|
|
Alkaline reagent test
|
Testsolution+fewdropsof NaOH solution
|
Intense yellow colour is obtained which turns to colourless on addition of few dropsofdilutedacid
|
Flavonoids are present
|
|
Lead acetatetest
|
Testsolution(alcoholicextract ofguavaleave)+Leadacetate solution
|
Yellowprecipitation occur
|
Quercetinand other Flavonoidsare
present
|
|
Sulfuricacidtest
|
Testsolution+sulfuricacid (66-80%)
|
Orangetored colour
|
Flavanones present
|
|
Ferricchloride test
|
Testsolution+ferricchloride solution (5%)
|
Brownishblueor green color
|
Hydrolysable tannins are
present
|
|
Gelatintest
|
Testsolution+1% gelatin
solution+10%NaCl solution
|
Whitebuffcolored ppt. formed
|
Tannins present
|
Table6 : Tests for Pomegranate Seed ethanolic extract
|
Test
|
Perform
|
Observation
|
Decision
|
|
Alkaline reagent test
|
Testsolution+fewdropsof NaOH solution
|
Yellow colour is obtained which turns to colourless on addition of few dropsofdilutedacid
|
Flavonoids are present
|
|
Lead acetate test
|
Testsolution(alcoholicextract ofguavaleave)+Leadacetate solution
|
Yellowprecipitation occur
|
Quercetinand other
Flavonoidsare present
|
|
Sulfuric acid test
|
Testsolution+sulfuricacid (66-80%)
|
Deepyellowcolor obtained
|
Flavonesand
flavanols present
|
|
Ferric chloride test
|
Testsolution+ferricchloride solution (5%)
|
Nobrownishblue or green color
obtained
|
Hydrolysable tannins are
not present
|
|
Gelatin test
|
Testsolution+1% gelatin
solution+10%NaCl solution
|
Nochanges/ppt. not formed
|
Tanninsare not present
|
Table7:Phytochemical analysis
|
Phyto-chemical tests
|
Pomegranate juice
|
Pomegranate peel ethanolic extract
|
Pomegranate peel water extract
|
Pomegranate seed ethanolic extract
|
|
Alkalinereagent test
|
+
|
+
|
+
|
+
|
|
Lead acetate test
|
+
|
+
|
+
|
+
|
|
Sulfuric acidtest
|
+
|
+
|
+
|
+
|
|
Ferric chloride test
|
+
|
+
|
+
|
-
|
|
Gelatin test
|
+
|
+
|
+
|
-
|
Extraction of the pomegranate juice:Extraction of the pomegranate juice:
To extract the arils and peel from the pomegranate fruit, wash it with distilled water and chop it by hand. Pomegranate juice must be manually extracted from the arilshell.
Preparation of the gel
- Take15 ml of pomegranate juice in a beaker.
- Heatitat40oCforsometime.
- Addit1g of Carbopol940 with continuous stirring.
- Added required amount of to copherol to it by heating on water bath.
- Now this mixture is mixed properly[27].
Evaluation of the pomegranate juice gel:
Viscosity:
The viscosity measurement of the herbal gel of pomegranate was performed with a Viscometer. The gel was rotated at 10, 20, 30, 40,50, and 60 rotations per minute. At each speed, the corresponding dial reading was noted.

Spreadability:
The spreadability of the gel was measured by spreading of 0.5g of the gel on a circle of 2cm diameter pre-marked on a glass plate and then a second glass plate was employed. Half kilogram of weight was permitted to rest on the upper glass plate for5 min. The diameter of the circle after spreading of the gel was determined[28]. The spreadability of gel was considered high by having a low spread of time. The therapeutic efficacy of gels depends on their spread. The gel spreading helps in the uniform application of the anti-ulcer gel to the skin, so the prepared gel will have a good spreadability and satisfy the ideal quality in topical application. Further more,this is considered as an important factor in patient compliance with treatment[29].
pHDetection:The pH of the gel is determined by using pH paper. The color is changed to green after putting the gel onto the paper. This shows that the gel would not produce skin irritation[30].
Appearance:Non transparent, Clear viscous gel without any microbeads.
Stability:Stable at room temperature and freezer. We stored it for 15 days in refrigerator and
at room temperature for the same days and no instability was seen.
Preliminary Phytochemical Screening of pomegranate juice gel:
Table8:Preliminary Phytochemical Screening of pomegranate juice gel
|
S.No
|
Phytoc-constituents
|
Observation
|
|
1.
|
Flavonoids
|
+
|
|
2.
|
tannins
|
+
|
DISCUSSION:
- Various problems and limitations were faced in different stages during performing this project work and are worth to mention.
- The extracts are very hygroscopic, so to avoid the stickiness’ and degradation of active moieties it must be stored in air tight container and kept it in freezer.
- During performing UV spectroscopy the temperature of the room must be maintained between 22°C to 25°C, exceeding which may cause variation in the UV spectroscopic data[31].
- While preparing the serial dilution for UV spectroscopy the solution must not kept open for long time, if done so the UV Spectroscopy data may getchanged.
- During filtering of the extract, it must be kept covered and when evaporation of the solvent temperature is kept low to save the active constituents.
- Use of double distilled water is highly recommended to avoid contamination and reaction with active constituents[32].
- While producing the Carbopol gel the use of magnetic stirrer with readily slow to high speed is recommended or hand mixing is recommended slowly toavoid unnecessary bubbles.
- The produced Carbopol gel is to be stored in air tight container away from light, preferably in storage facility within 4° to 8°C to avoid water loss and degradation respectively[33].
Future prospect:
- Total assh value will be determined
- Total phenolic content will be determined
- Total tannin content will be determined
- Total flavonoid content will be determined
- HPTLC will be done
Polyherbal capsules will be produce. The produced polyherbal capsules' description, uniformity of weight, disintegration time, moisture content, pH, Physiochemical parameters, and phytochemical research will all be standardized. For flavonoids, phenols, and tannins, a quantitative assessment of phytoconstituents will be performed. According to WHO guidelines, the polyherbal formulation's heavy metal analysis and microbiological load will be done and determined to be below acceptable limits. Utilizing the assay for inhibiting -amylase will be used to measure in vitro activity. According to OECD guidelines 423, an acute toxicity research will be conducted, and the polyherbalcapsules were foundto be safe upto 2000mg/Kg body weight.The therapeutic efficacy of the created and standardised polyherbal capsule will be assessed[34,35].
- Limitations:
- Effect of gels is relatively sustained and slower.
- The additives may cause irritation.
- Water content increases possibility of fungal or microbial attack in gel.
- Solvent loss from the formulation dries of gel[36].
- Flocculation in some gel causes an unstable gel
CONCLUSION:
The greatest characteristics and nutritional value of the herbal gel of crude pharmaceuticals were to be made using simple methods and with little equipments. For this herbal gel, additional research is needed. Pomegranate fruit (Punicagranatum) herbal gel is created and assessed. The gel's formulation has been optimised for high spreadability, good gelling, and PH maintenance. Pomegranate juice was used in the gel's formulation, and it was tested. Since the gels include antioxidant, anti-inflammatory, antiseptic, and antibacterial properties, it can be concludingthat they can be employed as multipurpose gels. So the work can conclude that the formulation was deemed to be satisfactory.
REFERENCE
- Kim ND, Mehta R, Yu W, Neeman I, Livney T, Amichay A, et al. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res Treat. 2002;71(3):203-17. doi:10.1023/A:1013820923895.
- Jia ZS, Tang MC, Wu JM. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555-9. doi:10.1016/S0308-8146(98)00102-2.
- Gonzalez-Barrio R, Borges G, Mullen W, Crozier A. Bioavailability of anthocyanins and ellagitannins following consumption of raspberries by healthy humans and subjects with an ileostomy. J Agric Food Chem. 2010;58(7):3933-9. doi:10.1021/jf100315d.
- Dinis TC, Madeira VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315(1):161-9. doi:10.1006/abbi.1994.1485.
- Ebrahimzadeh MA, Nabavi SM, Nabavi SF, Bahramian F, Bekhradnia AR. Antioxidant and free radical scavenging activity of H. officinalis L. var. angustifolius, V. odorata, B. hyrcana and C. speciosum. Pak J Pharm Sci. 2010;23(1):29-34. doi:10.3923/pjps.2010.29.34.
- Ebrahimzadeh MA, Nabavi SM, Nabavi SF. Correlation between the in vitro iron-chelating activity and polyphenol and flavonoid contents of some medicinal plants. Pak J Biol Sci. 2009;12(12):934-8. doi:10.3923/pjbs.2009.934.938.
- Fischer UA, Carle R, Kammerer DR. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril, and differently produced juices by HPLC-DAD-ESI/MSn. Food Chem. 2011;127(2):807-21. doi:10.1016/j.foodchem.2010.12.156.
- Qu W, Breksa AP, Pan Z, Ma H. Quantitative determination of major polyphenol constituents in pomegranate products. Food Chem. 2012;132(3):1585-91. doi:10.1016/j.foodchem.2011.11.136.
- Gülçin ?, Huyut Z, Elmasta? M, Aboul-Enein HY. Radical scavenging and antioxidant activity of tannic acid. Arab J Chem. 2010;3(1):43-53. doi:10.1016/j.arabjc.2009.12.008.
- Ozcelik B, Lee JH, Min DB. Effects of light, oxygen, and pH on the absorbance of 2,2-diphenyl-1-picrylhydrazyl. J Food Sci. 2003;68(2):487-90. doi:10.1111/j.1365-2621.2003.tb05699.x.
- Namiki M. Antioxidants/antimutagens in food. Crit Rev Food Sci Nutr. 1990;29(4):273-300. doi:10.1080/10408399009527528.
- Yu BP, Laganiere S, Kim JW. Influence of life-prolonging food restriction on membrane lipoperoxidation and antioxidant status. Basic Life Sci. 1988;49:1067-73. doi:10.1007/978-1-4615-9516-1_72.
- Yoshino M, Murakami K. Interaction of iron with polyphenolic compounds: Application to antioxidant characterization. Anal Biochem. 1998;257(1):40-4. doi:10.1006/abio.1997.2523.
- Wu C, Chen F, Wang X, Kim HJ, He GQ, Haley-Zitlin V, et al. Antioxidant constituents in feverfew (Tanacetum parthenium) extract and their chromatographic quantification. Food Chem. 2006;96(2):220-7. doi:10.1016/j.foodchem.2005.02.021.
- Fischer UA, Dettmann JS, Carle R, Kammerer DR. Impact of processing and storage on the phenolic profiles and contents of pomegranate (Punica granatum L.) juices. Eur Food Res Technol. 2011;233(5):797-816. doi:10.1007/s00217-011-1574-9.
- Fischer UA, Jaksch AV, Carle R, Kammerer DR. Influence of origin source, different fruit tissue, and juice extraction methods on anthocyanin, phenolic acid, hydrolysable tannin, and isolariciresinol contents of pomegranate (Punica granatum L.) fruits and juices. Eur Food Res Technol. 2013;237(2):209-21. doi:10.1007/s00217-013-1974-2.
- Türky?lmaz M, Tagi S, Dereli U, Özkan M. Effects of various pressing programs and yields on the antioxidant activity, antimicrobial activity, phenolic content, and colour of pomegranate juices. Food Chem. 2013;138(2-3):1810-8. doi:10.1016/j.foodchem.2012.11.094.
- Koppel K, Anderson EL, Chambers E 4th. Influence of processing on pomegranate (Punica granatum L.) juice flavor and aroma. J Sci Food Agric. 2015;95(5):1066-71. doi:10.1002/jsfa.6791.
- De Pasquale C, Catania P, Vallone M. Influence of the pressing system on pomegranate juice physical-chemical properties. Chem Eng Trans. 2017;58:433-8. doi:10.3303/CET1758073.
- Sorrenti V, Salerno L, Di Giacomo C, Acquaviva R, Siracusa MA, Vanella A. Imidazole derivates as antioxidants and selective inhibitors of nNOS. Nitric Oxide. 2006;14(1):40-5. doi:10.1016/j.niox.2005.08.002.
- Nafees M, Jaskani MJ, Ahmad I, Maryam, Ashraf I, Maqsood A, et al. Biochemical analysis of organic acids and soluble sugars in wild and cultivated pomegranate germplasm based in Pakistan. Plants. 2020;9(4):493. doi:10.3390/plants9040493.
- Ferrara G, Giancaspro A, Mazzeo A, Giove SL, Matarrese AMS, Pacucci C, et al. Characterization of pomegranate (Punica granatum L.) genotypes collected in Puglia region, Southeastern Italy. Sci Hortic. 2014;178:70-8. doi:10.1016/j.scienta.2014.07.036.
- Gözlekçi S, Saraço?lu O, Onursal E, Özgen M. Total phenolic distribution of juice, peel, and seed extracts of four pomegranate cultivars. Pharmacogn Mag. 2011;7(25):161-4. doi:10.4103/0973-1296.80681.
- Derakhshan Z, Ferrante M, Tadi M, Ansari F, Heydari A, Hosseini MS, et al. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice, and seeds. Food Chem Toxicol. 2018;114:108-11. doi:10.1016/j.fct.2018.02.023.
- Li Y, Guo C, Yang J, Wei J, Xu J, Cheng S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006;96(2):254-60. doi:10.1016/j.foodchem.2005.02.033.
- Orak HH, Yagar H, Isbilir SS. Comparison of antioxidant activities of juice, peel, and seed of pomegranate (Punica granatum L.) and inter-relationships with total phenolic, tannin, anthocyanin, and flavonoid contents. Food Sci Biotechnol. 2012;21(2):373-87. doi:10.1007/s10068-012-0059-2.
- Aviram M, Dornfeld L, Kaplan M, Coleman R, Gaitini D, Nitecki S, et al. Pomegranate juice flavonoids inhibit low-density lipoprotein oxidation and cardiovascular diseases: studies in atherosclerotic mice and in humans. Drugs Exp Clin Res. 2002;28(2-3):49-62.
- Cam M, Hi?il Y. Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 2010;123(3):878-85. doi:10.1016/j.foodchem.2010.05.011.
- Shin Y, Ryu JA, Liu RH, Nock JF, Watkins CB. Harvest maturity, storage temperature and relative humidity affect fruit quality, antioxidant contents and activity, and inhibition of cell proliferation of strawberry fruit. Postharvest Biol Technol. 2008;49(2):201-9. doi:10.1016/j.postharvbio.2008.01.008.
- Özgen M, Durgaç C, Serçe S, Kaya C. Chemical and antioxidant properties of pomegranate cultivars grown in the Mediterranean region of Turkey. Food Chem. 2008;111(3):703-6. doi:10.1016/j.foodchem.2008.04.043.
- Miguel G, Dandlen S, Antunes D, Neves A, Martins D. The effect of two methods of pomegranate (Punica granatum L.) juice extraction on quality during storage at 4°C. J Biomed Biotechnol. 2004;2004(5):332-7. doi:10.1155/S1110724304403022.
- Hasnaoui N, Jbir R, Mars M, Trifi M, Kamal-Eldin A, Melgarejo P, et al. Organic acids, sugars and anthocyanins contents in juices of Tunisian pomegranate fruits. Int J Food Prop. 2011;14(4):741-57. doi:10.1080/10942910903559919.
- Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48(10):4581-9. doi:10.1021/jf000404a.
- Elfalleh W, Tlili N, Nasri N, Yahia Y, Hannachi H, Chaira N, et al. Antioxidant capacities of phenolic compounds and tocopherols from Tunisian pomegranate (Punica granatum) fruits. J Food Sci. 2011;76(7):707-13. doi:10.1111/j.1750-3841.2011.02288.x.
- Adams LS, Seeram NP, Aggarwal BB, Takada Y, Sand D, Heber D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem. 2006;54(3):980-5. doi:10.1021/jf052005r.
- Aviram M, Dornfeld L, Rosenblat M, Volkova N, Kaplan M, Coleman R, et al. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr. 2000;71(5):1062-76. doi:10.1093/ajcn/71.5.1062.
- Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48(10):4581-9. doi:10.1021/jf000404a.
- Singleton VL, Rossi JA Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144-58.
- Pandidurai G, Vennila P. Studies on development of fruit powder from muskmelon (Cucumis melo L.) by using spray drier. Madras Agric J. 2018;105(4-6):215-9. doi:10.29321/MAJ.2018.000174.
- Prathyusha J, Yamani NS, Santhosh G, Aravind A, Naresh B. Formulation and evaluation of polyhedral face scrubber for oily skin in gel form. Int J Pharm Sci Drug Res. 2019;11(4):126-8. doi:10.25004/IJPSDR.2019.110403.
- Anwar E, Utami TD, Ramadon D. Transfersomal gel containing green tea (Camellia sinensis L. Kuntze) leaves extract: increasing in vitro penetration. Asian J Pharm Clin Res. 2017;10(5):326-30. doi:10.22159/ajpcr.2017.v10i5.17334.
- Nagoba SN, Shinde NG, Sakhare RS, Wadulkar RD, Deshmukh AY. Formulation and evaluation of herbal gel containing Punica granatum. Int J Innov Sci Eng Technol. 2019;6(7):74-9.
- Jadhav VD, Talele SG, Bakliwal AA, Chaudhari GN. Formulation and evaluation of herbal gel containing leaf extract of Tridax procumbens. Int J Pharma Res Health Sci. 2015;3(4):1045-9.
- Bhramaramba R, Sudheer Babu G, Divya Naga Deepthi C. Formulation and evaluation of herbal gel containing Terminalia chebula Retz. leaves extract. Scholars Acad J Pharm. 2015;4(3):172-6.
- Punasiya R, Yadav A, Krishna G, Pillai S. Formulation and evaluation of herbal gel containing extract of Hibiscus syriacus. Res J Pharm Technol. 2014;7(7):733-6


 SAIYED SELIM ALI*
SAIYED SELIM ALI*
 ABDUS SAMAD
ABDUS SAMAD
 HIMANGSHU SEKHAR MAJI
HIMANGSHU SEKHAR MAJI
 PINTU KUMAR DE
PINTU KUMAR DE













 10.5281/zenodo.14739419
10.5281/zenodo.14739419