Abstract
Momordica dioica, a member of the Cucurbitaceae family, is a perennial dioecious climber with tuberous roots widely distributed in India and other parts of the Indian subcontinent. The fruits, leaves, and tuberous roots of M. dioica are used as a folk remedy for diabetes mellitus in India. Phytochemical analysis of M. dioica fruits has revealed the presence of alkaloids, steroids, triterpenoids, flavonoids, glycosides, saponins, triterpenes, and other bioactive compounds. The fruit is rich in nutrients, containing high levels of carbohydrates, protein, lipids, fibre, minerals (calcium, iron, potassium, zinc, sodium), and vitamins (carotene, thiamin, riboflavin, niacin). M. dioica exhibits significant antioxidant activities, which may contribute to its potential in preventing and managing oxidative stress-related diseases such as diabetes. Oxidative stress, resulting from an imbalance between free radical generation and antioxidant defences, plays a crucial role in the pathogenesis of diabetes and its complications. M. dioica's antioxidant properties are attributed to its enzymatic and non-enzymatic antioxidant components, including catalase, glutathione system, thioredoxin system, superoxide dismutase, vitamin C, vitamin E, carotenoids, flavonoids, and polyphenols. These antioxidants neutralise reactive oxygen and nitrogen species (RONS), which can cause damage to lipids, DNA, and proteins when present in excessive levels. The beneficial role of M. dioica in diabetes management may be due to its ability to scavenge free radicals, reduce lipid peroxidation, and enhance antioxidant enzyme activities. Further research is needed to elucidate the specific mechanisms by which M. dioica exerts its antioxidant and anti-diabetic effects and to explore its potential as a natural therapeutic agent for diabetes and other oxidative stress-related disorders
Keywords
Momordicadioica, Cucurbitaceae, Phytochemicals, Antioxidantactivities, Medicinalproperties, Biogeography, Botanical description
Introduction

Fig.1
Cucurbitaceae is a plant family commonly known as melons, gourds or cucurbits and includes crops like cucumbers, squashes (including pumpkins), luffas, and melons (including watermelons). (Sivasudha et al 2012) the family is predominantly distributed around the tropics, where those with edible fruits were amongst the earliest cultivated plants in both the old and new world. Major genera under this family are Trichosanthes (100 species), Cayaponia (60 species), Momordica (47 species), Gurania (40 species), Sicyos (40 species) and Cucumis (34 species). This is one of the most genetically diverse groups of food plants in the plant kingdom. The plants belonging to this family are forst-sensitive, drought tolerant, and intolerant to wet and poorly drained soils, production of cucurbits seems to have increased over the time due to high demand and consumer. They are well known for the bitter taste due to the presence of phytochemical (alkaloid) and have a wide range of medicinal values.1-3A Momordica species is an annual or perennial climber that contains about 80 species (Raj et al 1993). This is generally found throughout India, Pakistan, Bangladesh, and also extends from Himalayas to Ceylon. Reported up to an altitude of 1500m in Assam, Garo hills of Meghalaya (Ram et al 2002) and Western Ghats, one of the mega diversity hotspots, hold a rich treasure of diversity in Momordica L, it comprises M. charantiavar. muricata, M. charantia var. charantia, M. dioica and M. sahyadrica (Joseph & Antony, 2008). The revival of interest in natural drugs started in last decade mainly because of the wide spread belief that green medicine is healthier than synthetic products. Nowadays, there is manifold increase in medicinal plant based industries. Due to the increase in the interest of medicinal plants throughout the world which are growing at a rate of 7-15% annually, despite the major advances in the modern medicine, the development of new drugs from natural products is still considered important. Medicinal plants as a possible therapeutic measure has become a subject of active scientific investigations. The Momordica species have been used in indigenous medical systems in various countries in Asia and Africa. Based on the indigenous knowledge, wild plant foods play a vital role in the complex cultural system of tribal people for reducing various disorders. Research has shown that many edible wild plants are rich in specific constituents, referred as phytochemical, which may have health promoting effects. So far no review has been covered from the literature encompassing valuable attributes of M. dioica in all dimensions. Its versatile utility as a nutritious vegetable, folk medicine and functional food ingredient provoked us to compile a comprehensive review of this multipurpose fruit on the distribution, nutritional attributes and phytochemical composition and its medicinal properties. Biogeography and Botanical description Based on the both historical literature and recent analysis Momordica dioica Roxb is a perennial dioecious climber with tuberous roots. Taxon Momordica dioica Roxb has been verified by US Department of Agriculture as member of family Cucurbitaceae, subfamily cucurbitoideae. Genus Momordica could perhaps refer to sculptured seed orknown as ‘Kakora’ in Gwalior Chambal Division of M.P., is supposed to have originated in Indo- Malayan region (Rashid, 1976 & Singh, 1990). In India, it is distributed widely from Himalayas to Southern peninsula and amongst other parts of Indian subcontinent including Pakistan, Bangladesh, Myanmar and Srilanka, growing wild and mostly cultivated for its fruit which is used as a vegetable (Sastri 1962, Singh et al 2009). The fruit is oval with soft spines; aerial part of the plant dies at the beginning of winter. Plant perennates through sprouting of tubers at the onset of survival of the plant and creates a big production loss. The species is cultivated by vegetative propagation method from underground tuberous roots. uneven appearance of fruit which look as if they have been bitten. The plant commonly known as ‘Kakora’ in Gwalior Chambal Division of M.P., is supposed to have originated in Indo- Malayan region (Rashid, 1976 & Singh, 1990). In India, it is distributed widely from Himalayas to Southern peninsula and amongst other parts of Indian subcontinent including Pakistan, Bangladesh, Myanmar and Srilanka, growing wild and mostly cultivated for its fruit which is used as a vegetable (Sastri 1962, Singh et al 2009). The fruit is oval with soft spines; aerial part of the plant dies at the beginning of winter. Plant perennates through sprouting of tubers at the onset of survival of the plant and creates a big production loss. The species is cultivated by vegetative propagation method from underground tuberous
roots.[1]
Phytochemical and Nutrient Study
The fruit of Momordica dioica contains ashes: 9.1%, crude protein: 5.44%, crude lipid: 3.25%, crude fibre: 22.9%, and carbohydrate: 59.31%. Its fruit has high energy value (288.25?kcal/100?g) in dry weight. Its mineral ranges (mg/100?g dry weight,) are: potassium (4.63), sodium (1.62), calcium (7.37), iron (5.04), and zinc (3.83) [14]. In another investigation, its nutritional value of per 100?g edible fruit is reported to contain 84.1% moisture, 7.7?g carbohydrate, 3.1?g protein, 3.1?g fat, 3.0?g fibre and 1.1?g minerals and small quantities of essential vitamins like carotene, thiamin, riboflavin and niacin [15].Ali and Deokule evaluated some of its micronutrient and secondary metabolites as follows: calcium: 0.5?mg/g, sodium: 1.5?mg/g, potassium: 8.3?mg/g, iron: 0.14?mg/g, zinc: 1.34?mg/g, protein: 19.38%, fat: 4.7%, total phenolic compound: 3.7?mg/g, phytic acid: 2.8?mg/g, and ash value: 6.7% [16]. Moreover, its fruit is recommended as nutritionally rich source of protein and good source of lipid, crude fibre, carbohydrate, iron, calcium, phosphorous. Additionally, it is the highest amount of carotene (162?mg/100?g of edible portion) container amongst the cucurbitaceous vegetables [17–19]. The ash content is reported as 3-4% containing a trace of manganese [20].Tirmizi et al. screened it as a potential source of chromium and zinc [21]. Whereas, Momordica dioica (peeled) contained 0.27?mg/kg of chromium and 4.91?mg/kg of zinc, Momordica dioica (unpeeled) contained 0.26?mg/kg of chromium and 11.0?mg/kg of zinc. The protein content of leaves and dry weight of aerial plant parts remained higher in male as compared to female defruited and monoecious plants [22]. The fruit contains higher amount of ascorbic acid and iodine [23, 24]. The presence of secondary metabolites of fruit including alkaloids, steroids, triterpenoids, and saponins was determined [25]. Among them, four compounds were isolated from ethyl acetate extract and five compounds were isolated from methanol extract consisting of alkaloids and flavonoids with NH and C=O functional groups, respectively. The alkaloids present in seed and root were called momordicin and Momordica foetida, respectively [26]. Phytochemical investigations summarised in Table 1 also showed the presence of lectins, ?-sitosterol, saponin glycosides, triterpenes of ursolic acid, hederagenin, oleanolic acid, ?-spinasterol, stearic acid, gypsogenin, momodicaursenol, and three new compounds named 3?-o-benzoyl-11-oxo-ursolic acid, 3?-o-benzoyl-6-oxo-ursolic acid, and 3-o-?-D-glucuronopyranosyl gypsogenin [2]
Table 1. Nutrient and phytochemical study of Momordica dioica as described in this paper
|
Classification
|
Compound
|
Extract or preparation
|
Reference
|
|
Crude protein
|
—
|
Quantitative analysis showed 5.44%
|
[14]
|
|
Protein
|
—
|
Quantitative analysis showed 3.1/100?g
|
[15]
|
|
—
|
Quantitative analysis showed 19.38%
|
[16]
|
|
Crude lipid
|
—
|
Quantitative analysis showed 3.25%
|
[14]
|
|
Fat
|
—
|
Quantitative analysis showed 3.1/100?g
|
[15]
|
|
—
|
Quantitative analysis showed 4.7%
|
[16]
|
|
Crude fibre
|
—
|
Quantitative analysis showed 22.9%
|
[14]
|
|
Carbohydrate
|
—
|
Quantitative analysis showed 59.31%
|
[14]
|
|
—
|
Quantitative analysis showed 7.7/100?g
|
[15]
|
|
Niacin
|
—
|
Not specified
|
[15]
|
|
Thiamin
|
—
|
Not specified
|
[15]
|
|
Carotene
|
—
|
Not specified
|
[15]
|
|
—
|
Quantitative analysis showed 162?mg/100?g of edible portion
|
[18, 19]
|
|
Ascorbic acid
|
—
|
Not specified
|
[24]
|
|
Potassium
|
—
|
Quantitative analysis showed 4.63?mg/100?g dry weight
|
[14]
|
|
—
|
Quantitative analysis showed 8.3?mg/g
|
[16]
|
|
Sodium
|
—
|
Quantitative analysis showed 1.62?mg/100?g dry weight
|
[14]
|
|
—
|
Quantitative analysis showed 1.5?mg/g
|
[16]
|
|
Calcium
|
—
|
Quantitative analysis showed 7.37?mg/100?g dry weight
|
[14]
|
|
—
|
Quantitative analysis showed 0.5?mg/g
|
[16]
|
|
Iron
|
—
|
Quantitative analysis showed 5.04?mg/100?g dry weight
|
[14]
|
|
—
|
Quantitative analysis showed 0.14?mg/g
|
[16]
|
|
Zinc
|
—
|
Quantitative analysis showed 3.83?mg/100?g dry weight
|
[14]
|
|
—
|
Quantitative analysis showed 1.34?mg/g
|
[16]
|
|
—
|
Not specified
|
[21]
|
|
—
|
Quantitative analysis showed 4.91?mg/kg (peeled), 11.0?mg/g (unpeeled)
|
[22]
|
|
Manganese
|
—
|
Not specified
|
[20]
|
|
Iodine
|
—
|
Not specified
|
[23]
|
|
Chromium
|
—
|
Quantitative analysis showed 0.27?mg/kg (peeled), 0.26?mg/kg (unpeeled)
|
[22]
|
|
—
|
Not specified
|
[21]
|
|
Phytic acid
|
—
|
Quantitative analysis showed 2.8?mg/g
|
[16]
|
|
Total phenolic compound
|
—
|
Quantitative analysis showed 3.7?mg/g
|
[16]
|
|
Alkaloids
|
—
|
Identified in ethyl acetate, methanol extract
|
[25]
|
|
Flavonoid
|
—
|
Identified in methanol, hexane extract
|
[25]
|
|
Steroids
|
—
|
Identified in ethyl acetate, methanol, aqueous extract
|
[25]
|
|
Saponins
|
—
|
Identified in methanol, aqueous extract
|
[25]
|
|
Triterpenoids
|
—
|
Identified in ethyl acetate, methanol, aqueous extract
|
[25]
|
|
Alkaloid
|
Momordicin
|
Identified in seed oil
|
[26]
|
|
Lectin
|
Anti-H-Lectin
|
Not specified
|
[30]
|
|
Alkaloid
|
Momordicafoetida
|
Not specified
|
[26]
|
|
Stearic acid
|
—
|
Identified in methanol extract
|
[31]
|
|
Steroid
|
?-spinasterol octadecanoate
|
Identified in methanol extract
|
[31]
|
|
?-spinasterol-3-O-?-D-glucopyranoside
|
Identified in methanol extract
|
[31]
|
Antioxidants are groups of compounds that neutralise free radicals and reactive oxygen species (ROS) in the cell (Abuajah et al. 2015). Antioxidant activity in food and beverages has become one of the most interesting features in the science community. These antioxidants provide protection against damage caused by free radicals played important roles in the development of many chronic disease including cardiovascular diseases, aging, heart disease, anaemia, cancer, inflammation[3]
The role of antioxidants in preventing oxidative stress-related diseases
Various sets of colonic microbiota cooperate to metabolise different phytochemicals. For instance, ellagic acids are metabolised to urolithins by bacteria like Clostridium spp., Ruminococcaceae, Eubacterium spp., Gordonibacter spp., and Ellagibacter isourolithinifaciens [4]. Daidzeins, isoflavones from soybeans, are instead converted into equals by another community of bacteria such as Streptococcus intermedia’s, Bacteroids ovatus, and Ruminococcus productus [5]. Lignans undergo modifications by diverse bacteria, including Clostridium scindens, Eggerthella lenta, Clostridiales, and Lactonifactor longoviformis [6]. Consequently, phytochemical metabolites can vary among individuals consuming the same food due to the individual differences in colonic microbiota composition. Many diseases heavily impacted by dietary factors entail oxidative damage as an initial occurrence or an early stage in the progression of the disease. Consequently, a significant emphasis in dietary disease prevention has been placed on antioxidant intervention. Over the past decade, a wealth of research, including numerous human intervention studies, has consistently highlighted the pivotal role of antioxidants, particularly phytochemicals, in mitigating the risk of chronic diseases [7].Traditionally, the beneficial role of antioxidants has been associated with curtailing the undesirable and uncontrolled production of reactive oxygen species, leading to a state known as oxidative stress. However, contemporary scientific understanding increasingly recognises that the mechanism of action of antioxidants in vivo may be far more intricate than previously thought.Beyond their role in mitigating oxidative stress, antioxidants and phytochemicals demonstrate multifaceted mechanisms that contribute to disease prevention. These mechanisms include the modulation of inflammatory pathways, the enhancement of cellular repair and regeneration, and interaction with signalling cascades involved in cell growth and apoptosis. Moreover, antioxidants exhibit the potential to influence epigenetic processes, altering gene expression patterns associated with disease susceptibility. Recent studies have illuminated the intricate interplay between antioxidants and the gut microbiota, revealing a symbiotic relationship. Antioxidants, particularly those derived from plant-based sources, can impact the amount and diversity of the gut microbiota, which, in turn, contribute to overall health and disease prevention. Additionally, antioxidants demonstrate neuroprotective effects and may play a crucial role in preserving cognitive function and preventing neurodegenerative diseases.
As research progresses, the understanding of the comprehensive impact of antioxidants and phytochemical on human health continues to evolve. Insights into their nuanced mechanisms of action open avenues for targeted interventions, personalised nutrition strategies, and the development of novel therapeutic approaches for a wide array of diseases influenced by dietary factors.
Phytochemical Constituents of Momordica dioica
- Qualitative phytochemical analysis of Momordica dioica root extracts:
Following the methods of qualitative analysis reveals the presence of phytochemical in root extracts of M. dioica prepared in petroleum ether and acetone, used for variety of ethnic medicinal uses. The alkaloids, terpenoids, phenols, carbohydrates and steroids are present in both petroleum ether and acetone extract of M. dioica. The flavonoids and saponins are present only in the acetone extract of M. dioica root and absent in petroleum ether extract, while the cardiac glycosides and tannins are present only in the petroleum ether extract of M. dioica root and are absent in acetone.[7]
- Quantitative phytochemical analysis of Momordica dioica root extracts
Following the methods of quantitative analysis of root extract reveals that the total alkaloid content estimated were 3.43 mg/g and 1.89 mg/g in petroleum ether and acetone respectively. 2.67 mg/g of flavonoids were present in acetonic root extract. The total phenolic content present in petroleum ether and acetone root extract were 2.83 and 1.69 mg/g respectively. The total tannins estimated in petroleum ether root extract was 3.13 mg/g, while the total saponins estimated in[8]
Table-2
|
Sr.no.
|
Phytochemical constituents [mg/g]
|
Petroleum ether (mg/g
|
Acetone (mg/g)
|
|
1
|
Alkaloids
|
3.43
|
1.89
|
|
2
|
Flavonoids
|
--
|
2.67
|
|
3
|
Phenols
|
2.83
|
1.69
|
|
4
|
Phenols
|
2.83
|
1.69
|
|
5
|
Saponin
|
--
|
2.12
|
Analytical Methods Used in Phytochemical Identification
Steps Involved In Plant Collection
2.1.Collection of Plants Plants under consideration may be collected either from wild forests or from herbariums. When plants are collected from wild, there is a risk that they have been incorrectly identified. The major advantage of wildlife plants is that they will not contain any pesticides. After the plants are collected from wild or from herbarium they have to be processed for cleaning in order to prevent the deterioration of phytochemical present in plants.
2.2.Cleaning of Plants After plants collection they have to be cleaned properly. The cleaning process may involve the following steps. Cleaning, washing, peeling or stripping leaves from stems. Cleaning has to be done by hands in order to get better results.
2.3.Drying The main purpose of drying is to remove the water content from plants so that the plants can be stored. Plants have to be dried immediately as soon as the plants collection or this will lead to spoilage of plant materials. The drying consists of two methods. Drying can be done either by natural process or by artificial process.
2.3.1. Natural Process Natural process includes sun- drying. Sometimes plants are placed on drying frames or on stands, to be air-dried in barns or sheds. But this may take few weeks for complete drying. The time depends on temperature and humidity.
2.3.2. Artificial Drying Artificial drying can be done with the help of artificial driers. This process will reduce the drying time to several hours or minutes. The common method that is followed in drying medicinal plants is warm-air drying. In this process plants are placed in the plates of drier on which warm air is blown. This method is mainly applicable to fragile flower and leaves and this requires large number of workers since loading and unloading of plants has to be done manually. 2.4.Powdering After complete drying of plants they have to be powdered well for further analysis [9]
METHODS OF EXTRACTION
3.1. Plant Tissue Homogenisation Plant tissue homogenisation in solvent has been widely used by researchers. Dried or wet, fresh plant parts are grinder in a blender to fine particles, put in a certain quantity of solvent and shaken vigorously for 5 - 10 min or left for 24 h after which the extract is filtered. The filtrate then may be dried under reduced pressure and re-dissolved in the solvent to determine the concentration. Some researchers however centrifuged the filtrate for clarification of the extract.
3.2 Serial Exhaustive Extraction It is another common method of extraction which involves successive extraction with solvents of increasing polarity from a non-polar (hexane) to a more polar solvent (methanol) to ensure that a wide polarity range of compounds could be extracted. Some researchers employ Soxhlet extraction of dried plant material using organic solvent. This method cannot be used for thermolabile compounds as prolonged heating may lead to degradation of compounds.
3.3. Soxhlet Extraction Soxhlet extraction is only required where the desired compound has a limited solubility in a solvent, and the impurity is insoluble in that solvent. If the desired compound has a high solubility in a solvent then a simple filtration can be used to separate the compound from the insoluble substance. The advantage of this system is that instead of many portions of warm solvent being passed through the sample, just one batch of solvent is recycled. This method cannot be used for thermolabile compounds as prolonged heating may lead to degradation of compounds
3.4. Maceration In maceration (for fluid extract), whole or coarsely powdered plant- drug is kept in contact with the solvent in a stoppered container for a defined period with frequent agitation until soluble matter is dissolved. This method is best suitable for use in case of the thermolabile drugs
3.5. Decoction This method is used for the extraction of the water soluble and heat stable constituents from crude drug by boiling it in water for 15 minutes, cooling, straining and passing sufficient cold water through the drug to produce the required volume.
3.6. Infusion It is a dilute solution of the readily soluble components of the crude drugs. Fresh infusions are prepared by macerating the solids for a short period of time with either cold or boiling water.
3.7. Digestion This is a kind of maceration in which gentle heat is applied during the maceration extraction process. It is used when moderately elevated temperature is not objectionable and the solvent efficiency of the menstrual is increased thereby.
3.8. Percolation This is the procedure used most frequently to extract active ingredients in the preparation of tinctures and fluid extracts. A percolator (a narrow, cone-shaped vessel open at both ends) is generally used. The solid ingredients are moistened with an appropriate amount of the specified menstrual and allowed to stand for approximately 4 h in a well closed container, after which the mass is packed and the top of the percolator is closed. Additional men-strum is added to form a shallow layer above the mass, and the mixture is allowed to macerate in the closed percolator for 24 h. The outlet of the percolator then is opened and the liquid contained therein is allowed to drip slowly. Additional men-strum is added as required, until the percolate measures about three-quarters of the required volume of the finished product. The Marc is then pressed and the expressed liquid is added to the percolate. Sufficient men-strum is added to produce the required volume, and the mixed liquid is clarified by filtration or by standing followed by decanting.
3.9. Sonication
The procedure involves the use of ultrasound with frequencies ranging from 20 kHz to 2000 kHz; this increases the permeability of cell walls and produces cavitation. Although the process is useful in some cases, like extraction of rauwolfia a root, its large-scale application is limited due to the higher costs. One disadvantage of the procedure is the occasional but known deleterious effect of ultrasound energy (more than 20 kHz) on the active constituents of medicinal plants through formation of free radicals and consequently undesirable changes in the drug molecules[12]
4.Qualitative And Quantitative Analysis Of Phytochemical
4.1.Preliminary Qualitative Analysis
1. Test for Alkaloids a. Mayer’ s test
To a few ml of plant sample extract, two drops of Mayer?s reagent are added along the sides of test tube. Appearance of white creamy precipitate indicates the presence of alkaloids.[6] b. Wagner’s test A few drops of Wagner?s reagent are added to few ml of plant extract along the sides of test tube. A reddish- Brown precipitate confirms the test as positive.
2. Test for Amino acids
The extract (100 mg) is dissolved in 10 ml of distilled water and filtered through Whatmann No. 1 filter paper and the filtrate is subjected to test for Amino acids. a. Ninhydrin test Two drops of ninhydrin solution (10 mg of ninhydrin in 200 ml of acetone) are added to 2 ml of aqueous filtrate. Appearance of purple colour indicates the presence of amino acids.
3. Test for Carbohydrates
Molisch’ s test To 2 ml of plant sample extract, two drops of alcoholic solution of ?- naphthol are added. The mixture is shaken well and few drops of concentrated sulphuric acid is added slowly along the sides of test tube. A violet ring indicates the presence of carbohydrates. b. Benedict’ s test To 0.5 ml of filtrate, 0.5 ml of Benedict?s reagent is added. The mixture is heated on a boiling water bath for 2 minutes. A characteristic coloured precipitate indicates the presence of sugar.[12]
4. Test for Fixed oils and Fats
a.Spot test A small quantity of extract is pressed between two filter papers. Oil stain on the paper indicates the presence of fixed oils. b. Saponification test A few drops of 0.5 N alcoholic potassium hydroxide solution is added to a small quantity of extract along with a drop of phenolphthalein. The mixture is heated on a water bath for 2 hours. Formation of soap or partial neutralisation of alkali indicates the presence of fixed oils and fats.
5. Test for Glycosides
For 50 mg of extract is hydrolysed with concentrated hydrochloric acid for 2 hours on a water bath, filtered and the hydrolysate is subjected to the following tests.[13]
Qualitative and quantitative Analysis
Qualitative and quantitative analysis of phytochemical can be done using Gas Chromatography Mass Spectroscopy (GCMS). GCMS can be applied to solid, liquid and gaseous samples. First the samples are converted into gaseous state then analysis is carried out on the basis of mass to charge ratio. High Performance Liquid Chromatography is applicable for compounds soluble in solvents. High performance thin layer chromatography is applicable for the separation, detection, qualitative and quantitative analysis of phytochemical.
1. Gas Chromatography
Gas chromatography is applicable for volatile compounds. In this method, species distribute between a gas and a liquid phase. The gas phase is flowing and the liquid phase is stationary. When the sample molecules are in liquid phase they are stationary. The rate of migration depends on how much of chemical species is distributed into liquid phase. Higher the percentage of material in the gaseous state faster will be the migration. The species which distributes itself 100% in the stationary state will not migrate. If a sample distributes itself in both phases, it will migrate at an intermediate rate. This gas chromatography gives the total amount of vapour. Thus it is most widely used for quantitative analysis. [32]
2. High Performance Liquid Chromatography: (HPLC)
HPLC is also known as High- Pressure Liquid Chromatography. This separates compounds on the basis of their interaction with solid particles of a tightly packed column and the solvent of the mobile phase. High pressures of up to 400 bars are required to elute the analyse through the column before they pass through detector. HPLC is useful for compounds that cannot be vaporised or that decompose under high temperatures. HPLC provides both quantitative and qualitative analysis in a single operation.[33]
3. High Performance Thin Layer Chromatography: (HPTLC)
High Performance Thin layer Chromatography is a modified version of thin layer chromatography. High Performance Thin layer Chromatography is planer chromatography where separation of sample components is done on high performance layers with detection and acquisition using an advanced work- station. These high performance layers are pre-coated with a sorbent of particle size 5-7 microns and a layer thickness of 150-200 microns. The reduction in the thickness of the layer and the particle size results in increasing the plate efficiency along with nature of separation. HPTLC is suitable for qualitative, quantitative and micro-preparative chromatography.
4. Optimum Performance
Laminar Chromatography: (OPLC) OPLC combines the advantages of TLC and HPLC. The system separates about 10-15 mg samples, with simultaneous processing of up to 4 or 8 samples at a time depending on the model. In OPLC a pump is used to force a liquid mobile phase through a stationary phase, such as silica or a bonded-phase medium. The OPLC column housing structure allows flat planar columns to be used in the same way as cylindrical glass or stainless steel ones. The flat column is pressurised up to 50 bars, and mobile phase is forced through it at constant linear velocity via a solvent delivery pump. The work station includes all of the modules required for effective separation of the compound sample of interest, including two 96- well plate sample holders and automated sampling system that withdraws a sample from each well and places it on the OPLC planar sorbent bed, a solvent delivery system including a mobile phase degassed and pump, OPLC purification unit, a four channel diode array detector to monitor the eluent and fraction collector to six 96- well plates to hold the separated compounds. [34]
Phytochemical’s:
Satya Narayan Talukdar and Mohammad Nazir Hossain(2014),and Venkateshwarlu M. et al (2017) worked on phytochemical analysis of Momordica dioica Fruits. Phytoconstituents of Momordica dioica are traces of alkaloids, steroids, triterpenoids, flavonoids, glycosides, saponins, triterpenes of urisolic acid dark brown-semidrying oil and saturated fatty acids, ascorbic acids , vitamin A , thiamine, riboflavin’s, niacin, protein carbohydrates, lectins, ascorbic acids, carotenes, bitter principles, oleanoic acid, stearic acid, gypsogenin, alpha-spiranosterol hederagenin, momordicaursenol. The alkaloid present in this plants seed called momordicin. Other alkaloid present in root called momordica foetida. The fruit of Momordica dioica contains carbohydrate: 59.31%, ashes: 9.1%, crude protein: 5.44%, crude lipid: 3.25% and crude fibre: 22.9%. These fruit has high energy value (288.25 kcal/100 g) in dry weight. These fruit mineral ranges (mg/100 g dry weight,) are: calcium (7.37), iron (5.04), potassium (4.63), zinc (3.83) and sodium (1.62). Its nutritional value of Momordica dioica per 100 g edible fruit is reported to contain 84.1% moisture, 7.7 g carbohydrate, 3.1 g protein, 3.1 g fat, 3.0 g fibre and 1.1 g minerals and small quantities of essential vitamins like carotene, thiamin, riboflavin and niacin[35]
Fruits:
The Fruit of Momordica plant are green and generally used as vegetable by local people.It is shown in photoinage no.1. This fruit also used as medicine on health problem. Fruit are diuretic increased production of urine, salexiteric, hepatoprotective, and used to treat certain venomous bites. It is also used to cure asthma, leprosy, excessive salivation, prevent the inflammation caused by lizard, snake bite, elephantiasis, fever, mental disorders, digestive disorders and troubles of heart and to treat discharge from mucous membrane. Fresh fruit juice is prescribed for hypertension. The fruit is cooked in a small amount of oil and consumed for treating diabetic patient; juice of the young fruits can be applied on skin for pimples and acne to keep the skin healthy. Seeds are roasted and taken for eczema and other skin problems. This fruit is considered as a low-calorie, so add it to your monsoon diet for reduce extra fat
Leaves:
The leaves of Momordica dioica plant are used in India as a folk remedy for diabetic patient.It is shown in photo-image no.2. Juice of leaves worked as pain relief, the juice of the leaves mixed with coconut, pepper, red sandalwood in order to form an ointment and applied to the head to relieve pain in the head. In skin disease leaf paste of this plant more effective. Leaf paste is used both orally and external application
Roots:
Roots of the Momordica dioica are full of medicinal value. Spine guard tubers are styptic in nature and toasted roots or its powder is used in bleeding piles. The local people its tuber are used with honey in headache problem. The root juices of Momordica dioica plant are also used to relieve inflammation caused by lizard excretion. They are also used in mental disorder and digestive ailments. The most important use of Momordica dioica root is treat diabetes, root juice in the dose of 50ml once a day in empty stomach is given to treat diabetes. In diabetes, tuber powder along with Vanga bhasma is given. It causes vomiting in excess dose. The root powder applied externally softens skin and reduces excess sweating. In snake bite and scorpion stings root paste of spine guard is applied externally or nasal instillation is done. In any difficulty in urination, root along with milk is given to drink.
- Leaves Pain in head, skin disease, diabetic. Fever, eyes disease.
- Fruits Pimples treatment, remedy for diabetic, hypertension, eyes disease.
- Roots Treat bleeding piles, headache, relieves inflammation, used in mental disorder and digestive ailments, skin treatment and reduce excess sweating, snake bite and scorpion stings. Difficulty in urination.
Phytochemical and Nutrient Study
The fruit of Momordica dioica contains ashes: 9.1%, crude protein: 5.44%, crude lipid: 3.25%, crude fibre: 22.9%, and carbohydrate: 59.31%. Its fruit has high energy value (288.25?kcal/100?g) in dry weight.
. Antioxidant Activities of Momordica dioica.
Diabetes mellitus (DM) is a metabolic disorder characterised mainly by chronic hyperglycaemia resulting from defects in insulin secretion and/or its action. Chronic hyperglycaemia leads to improper regulation of carbohydrate, protein, and lipid metabolism and contributes to the progression of micro and macro-vascular complications.[36] Oxidative stress has been suggested as mechanism underlying DM and diabetic complications.[37]
Free radicals are continuously produced in the body as a result of normal metabolic processes and interaction with environmental stimuli. Oxidative stress results from an imbalance between radical-generating and radical-scavenging systems that has increased free radical production or reduced activity of antioxidant defences or both. Role of oxidative stress in the pathogenesis of DM is suggested not only by oxygen free-radical generation but also due to non-enzymatic protein glycosylation, auto-oxidation of glucose, impaired glutathione metabolism, alteration in antioxidant enzymes, and formation of lipid peroxides.[38]It is well-known that superoxide anion is the primary radical formed by the reduction of molecular oxygen that may lead to secondary radicals or reactive oxygen species (ROS) such as hydrogen peroxide and hydroxyl radical. ROS are part of the defence mechanism against infection, but excessive generation of free oxygen radicals may damage tissues (Ahmed, 2005). Co-operative defence systems that protect the body from free radical damage include enzymatic and non-enzymatic antioxidants.[39] Enzymatic antioxidants namely glutathione-s-transferase (GST), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) and non-enzymatic antioxidants such as vitamins C and E, reduced glutathione (GSH), and ceruloplasmin (CP) play an important role in preventing tissue damage due to the formation of free radicals. High levels of glucose or glycated proteins enhances lipid peroxidation.[40] The efficiency of antioxidant defence system altered in DM and, therefore, the ineffective scavenging of free radicals may play a crucial role in pathophysiology of DM
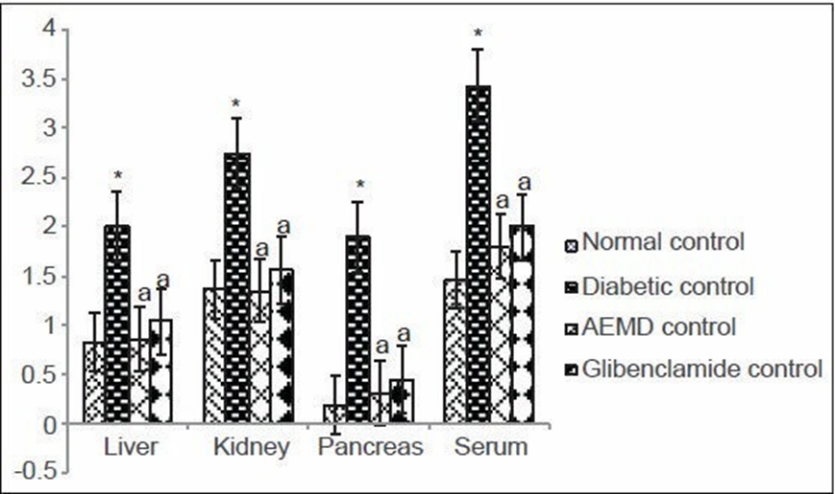
Fig.2
RONS (Reactive Oxygen and Reactive Nitrogen Species)
ROS and reactive nitrogen species (RNS) are collectively called RONS. These types of free radicals are mainly produced by cellular enzymes such as NADPH-oxidase, myeloperoxidase and nitric oxide synthase (NOS).Reactive nitrogen species (RNS) act with reactive oxygen species (ROS) to induce nitrosative stress condition. In animal cells, RNS production starts with the reaction of superoxide (O2•?) with nitric oxide (NO•) to form peroxynitrite (ONOO?), which is a highly reactive species with the ability to damage lipids, DNA bases, proteins, thiols, etc. Since generation of RNS is linked to ROS, it is not surprising that scavengers and antioxidants could reduce the formation and activity of RNS and thus nitrosative stress condition.The evolutionary role of low concentrations of RONS is known in the immune system where these radicals will eliminate foreign entities within cells to benefit the organism [41]. A growing body of evidence suggests an important role of RONS in intracellular signalling pathways and in processes such as regulating vascular tone, insulin synthesis, hypoxia-inducible factor (HIF) activation, cell proliferation, differentiation and migration42,43]. In contrast, excessive levels of these free radicals may cause harmful effects on biological structures as discussed previously [44]. Indeed, one of the most susceptible RONS targets is the proteome, the vital machinery for cellular protein homeostasis.Despite the harmful effects of RONS, a number of proteins are resistant to oxidative stress because of ROS-mediated thiol modifications particularly in cysteine residues. The thiol modification is involved in the regulation of protein function and structure under stress conditions [45]. Depending on the cell’s redox-state, the thiol group of the cysteine residue can be either reduced to a free thiol (SH) by the antioxidant defence system or converted to other oxidative post-translational modifications (Ox-PTMs) [46].Protein oxidation has been well shown in brains undergoing neurodegeneration. For example, protein carbonyls[47,48] and 3-nitrotyrosine [49] are observed in Alzheimer’s disease (AD) and Parkinson’s disease (PD) brains [50]. Indeed, proteomics approaches in AD brains have revealed a number of oxidized proteins including creatine kinase BB (CK), glutamine synthase (GS), ubiquitin carboxy-terminal hydrolase L-1 (UCHL1), ?-enolase, and dihydropyrimidinase related protein 2 (DRP2) [52,55]. Creatine kinase (BB isoform), ?-enolase, and triosephosphate isomerase are involved in energy production processes in cells and it seems that a reduction in ATP (Adenosine triphosphate) levels may initiate the degeneration of neurons in AD brain [56]. Proteomic studies further revealed that ?-synuclein, a hallmark protein of PD (which lacks tryptophan and cysteine residues), undergoes methionine oxidation to form methionine sulfoxides in the substantia nigra in PD brains. Methionine oxidation has inhibitory effects on protein fibrillation. This can result in the subsequent ?-synuclein aggregation, which in turn may influence the onset and progression of PD [57].
Scavengers
The study of scavengers and their beneficial role within imbalanced redox situations may provide new insights into new therapeutic strategies for oxidative stress-related diseases. Natural antioxidant systems are categorised into enzymatic and non-enzymatic antioxidant groups. The enzymatic group include a number of enzymes such as catalase, the enzymes of glutathione thioredoxin system and superoxide dismutase (SOD)[58]. Catalases exist in eukaryotic peroxisomes and catalyse the conversion of H2O2 into water and oxygen in the presence of iron or manganese cofactors[59]. The glutathione system comprises of three enzymes, glutathione reductase, glutathione peroxides and glutathione S-transferases, that contribute in breakdown of H2O2 and hydroperoxides using selenium as the cofactor [60,61]. The thioredoxin system comprises of thioredoxin protein and thioredoxin reductase and acts as a scavenging factor for ROS, a phenomenon that makes other proteins stable in a reduced state[62]. Superoxide dismutases are also antioxidant enzymes that work with cooper/zinc in the cytosol and manganese in mitochondria catalysing the breakdown of superoxide anions into oxygen and H2O2 [63,64].
The non-enzymatic group contains a number of antioxidants that act directly on oxidative agents and are acquired from dietary sources. This group includes vitamin C (Ascorbate), vitamin E (-tocopherol), carotenoids, flavonoids, polyphenols etc. Ascorbate plays an important role in detoxification of peroxyl and hydroxyl radicals, superoxide, singlet oxygen, and peroxynitrite in many organs particularly in the brain[65]. Vitamin E can interact with ascorbate and protect brain cells during stress conditions[66,67].There are also antioxidants that are produced by cells and chelate and/or bind to redox metals thus protect the cells against oxidative stress indirectly[68]. Melatonin, one of the first examples of natural scavengers is known to be involved in neutralisation of hypochlorous acid and also detoxify a wide range of environmental and chemical agents including H2O2, hydroxyl radical (OH•), peroxyl radicals (ROO•), singlet oxygen (1O2), and reactive nitrogen species (RNS) such as nitric oxide radical (NO•) and peroxinitrite (ONOO?)[69,70,71]. There are several mechanism in which melatonin is involved in oxidative stress protection including electron donation, hydrogen donation, addition, substitution, nitration and repair[72]. Other endogenously produced antioxidants are ubiquinol (coenzyme Q), a product of the mevalonate pathway [73]and glutathione synthesised from the amino acids L-cysteine, L-glutamic acid, and glycine.
Some scavengers such as 3,4-dihydroxyphenylalanine (L-DOPA) are currently used for therapeutic purposes for PD treatment [74,75]. Although widely used as a symptomatic relief therapeutic for PD there are conflicting data on its negative effect on dopaminergic neurone and its positive effects on motor system [76]. Another scavenger is Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one) that acts as a free radical detoxifier frequently used in acute ischemic stroke [77]. The scavenging role of Edaravone against oxygen and hydroxyl radicals, and its inhibitory role in lipid peroxidation/lipoxygenase pathways, suggests that Edaravone may be a potential therapeutic for oxidative-induced diseases [78,79,].
The Importance of Antioxidant Activity for the Health-Promoting Effect of Lycopene
Lycopene
Lycopene is a compound of red and orange fruit and vegetables, like tomato, watermelon, papaya, pink guava, carrot, rose-hip, apricot, pink grapefruit, and
pumpkin[80]. It is responsible for their red and orange colour due to light absorption with a maximum wavelength of ? = 444, 470, and 502 nm [81]. It takes part in the process of photosynthesis and protects plants from damage caused by overexposure to light. It is also an essential intermediate in the synthesis of beta-carotene and xanthophylls [82]. However, lycopene is also found in some plants that have other colours, such as parsley and asparagus [83].
The most common source of lycopene in diets are tomatoes and products containing tomatoes More than 85% of this ingredient in our diet comes from these sources. Tomatoes are also the cheapest source for lycopene production. The tomato-based products are a better source of this compound than raw tomatoes Different varieties of tomatoes, as well as other fruits and vegetables, contain different amounts of this ingredient[84].The amount of this carotenoid is affected by various factors, such as the degree of maturity of the plant material, fruit variety, light, temperature, climate, irrigation, location of plantation, soil quality, processing, and conditions of storage When the temperature exceeds 35 °C, the amount of lycopene decreases because it is converted to beta-carotene. The lycopene content can increase by about 36% when grown in soil containing the necessary microorganisms. The amount of this carotenoid is higher in ripe fruits because they contain less chlorophyll Light and oxygen have the greatest impact on storage and processing. Its synthesis in tomatoes is promoted via supplementation with red, far-red, and blue light []. The content of lycopene in tomato puree shows the highest stability (with a total loss of 20%). It has high stability in comparison with other substances, such as ascorbic acid, kaempferol, and quercetin, and also after multiple sterilisation and evaporation cycles. Its stability may be related to the presence of, e.g., ascorbic acid and phenolic compounds in tomatoes, and also to the influence of these compounds on the inhibition of the process of isomerization and auto-oxidation of lycopene. They ensure greater stability compared to pure lycopene [85,86]
Photochemical Constituents
Terpenoids: A large class of secondary metabolites that can have anti-inflammatory, anticancer, and cardiovascular disease reducing biological activities
Phenolic compounds: The accumulation of phenolic compounds in Momordica dioica can be studied at different stages of maturity
Health Implications of Anti Oxidant Activity
The present study demonstrated that aqueous extract of Momordica dioica possess potent antioxidant activity. Protection and regeneration of pancreatic ?-cell function and enhancement in insulin secretion may be one of the reasons for its anti-diabetic activity of M. dioica fruits.
TLDRThe protective role of AEMD on liver, kidney, and pancreas in severe diabetic rats justifying support for its anti-diabetic use in folk medicine is shown.
Increase in the levels of TBARS, HP and decrease in the levels of non-enzymatic antioxidants and activity of enzymatic antioxidants was observed in liver, kidney, pancreas, and serum of diabetic rats when compared with normal healthy rats. TBARS and HP levels were reduced while non-enzymatic and enzymatic antioxidant enzymes activity was increased in AEMD and glibenclamide-treated rats. Furthermore, histological examination of liver, kidney, and pancreas of diabetic rats showed degenerative changes. AEMD treatment for 21 days rejuvenated liver, kidney, and pancreas histoarchitecture
Phytochemical-Antioxidant Relationship
Phytochemicals are naturally occurring chemical compounds found in plants. They give plants their vibrant colours, distinct flavours, and, most importantly, their protective properties. Many phytochemicals act as antioxidants, helping to protect our bodies from oxidative stress.
Synergistic Effects of Phytochemicals:
- Momordica dioica, also known as spiny gourd or teasle gourd, is a plant rich in various phytochemicals with potential health benefits. These phytochemicals often exhibit synergistic effects, meaning their combined action is greater than the sum of their individual effects.
- Key Phytochemicals and Their Potential Synergistic Interactions:
- Flavonoids: These compounds are known for their antioxidant and anti-inflammatory properties. In Momordica dioica, flavonoids like quercetin and kaempferol may work synergistically with other phytochemicals to enhance their effects.
- Terpenoids: These compounds, including triterpenoids and saponins, have been shown to possess anti-diabetic, antitumor, and immunomodulatory activities. In combination with other phytochemicals, they may amplify these effects.
- Phenolic Acids: These compounds, such as caffeic acid and chlorogenic acid, have antioxidant and anti-inflammatory properties. They may work synergistically with flavonoids and terpenoids to enhance their protective effects.
- Potential Synergistic Effects and Health Benefits:
- Anti-diabetic Effects: Studies suggest that the combination of phytochemicals in Momordica dioica may help regulate blood sugar levels by improving insulin sensitivity and glucose uptake.
- Antioxidant and Anti-inflammatory Effects: The synergistic action of antioxidants in Momordica dioica may help protect cells from oxidative damage and reduce inflammation, which are implicated in various chronic diseases.
- Anticancer Effects: Some studies suggest that the combination of phytochemicals in Momordica dioica may have anticancer properties by inhibiting tumor growth and promoting apoptosis (programmed cell death) in cancer cells.
- Immunomodulatory Effects: The synergistic action of certain phytochemicals may help modulate the immune system, enhancing its ability to fight infections and diseases.
- Discussion of how multiple phytochemicals in *Momordica dioica* may work synergistically to enhance antioxidant activity.
- Flavonoids:
- Quercetin and Kaempferol: These flavonoids are potent antioxidants that can scavenge free radicals directly.
- Synergistic Effect: When combined, they may exhibit broader antioxidant activity, covering a wider range of free radical species.
- Terpenoids:
- Triterpenoids and Saponins: These compounds possess antioxidant properties and can also modulate antioxidant enzymes.
- Synergistic Effect: Triterpenoids might enhance the membrane-stabilizing effects of saponins, indirectly protecting cells from oxidative damage.
- Phenolic Acids:
- Caffeic Acid and Chlorogenic Acid: These compounds are known for their antioxidant and anti-inflammatory properties.
- Synergistic Effect: They can work in concert with flavonoids to scavenge free radicals more efficiently and reduce oxidative stress.
- Mechanisms of Synergistic Antioxidant Activity:
- Enhanced Free Radical Scavenging: Different phytochemicals may target different types of free radicals, leading to more comprehensive antioxidant protection.
- Modulation of Antioxidant Enzymes: Some phytochemicals can upregulate the activity of endogenous antioxidant enzymes like superoxide dismutase (SOD) and catalase, amplifying the body's natural defense against oxidative stress.
- Reduced Oxidative Stress Markers: The combined action of multiple phytochemicals may lead to a significant reduction in oxidative stress markers such as malondialdehyde (MDA) and protein carbonyl content.
Potential Health Benefits:
- Protection against Chronic Diseases: The synergistic antioxidant activity of Momordica dioica phytochemicals may help prevent chronic diseases linked to oxidative stress, such as cardiovascular disease, cancer, and neurodegenerative disorders.
- Improved Overall Health: By reducing oxidative damage, these phytochemicals may contribute to improved overall health and well-being.
Influence of Environmental and Extraction
Geographical Location and Climate:
- Sunlight: Higher sunlight exposure can lead to increased production of secondary metabolites like flavonoids and phenolic compounds, which contribute to antioxidant activity.
- Temperature: Optimal temperatures can enhance plant growth and metabolite synthesis, while extreme temperatures can stress the plant, potentially affecting phytochemical content.
- Rainfall: Adequate rainfall is essential for plant growth and nutrient uptake, which can influence the overall phytochemical profile. However, excessive rainfall can lead to leaching of nutrients and reduce metabolite production.
- Soil Composition: Soil type and nutrient availability can significantly impact plant growth and metabolite accumulation. For example, plants grown in nutrient-rich soils may exhibit higher levels of certain phytochemical.
- Altitude: Plants grown at higher altitudes may experience different environmental conditions, such as lower temperatures and higher UV radiation, which can influence their phytochemical profile.
- Solvent Type: Different solvents (e.g., water, ethanol, methanol) have varying polarities and can extract different classes of phytochemical. For example, polar solvents like water and ethanol are more effective in extracting polar compounds like flavonoids and phenolic acids, while non-polar solvents like hexane are better at extracting non-polar compounds like terpenes.
- Temperature: Higher temperatures can increase the rate of extraction but may also degrade heat-sensitive compounds.
- Time: Longer extraction times can increase the yield of phytochemical but may also lead to degradation or oxidation of certain compounds.
- Particle Size: Smaller particle sizes increase the surface area available for solvent contact, leading to faster and more efficient extraction.
- Solid-to-Solvent Ratio: The ratio of plant material to solvent can influence the concentration of extracted compounds.Impact on Phytochemical Profile and Antioxidant Capacity:
- Increased Antioxidant Activity: Plants grown in environments with higher sunlight exposure, optimal temperatures, and adequate rainfall, and extracted using appropriate solvents and conditions, may exhibit higher levels of antioxidants.
- Enhanced Phytochemical Diversity: Different environmental and extraction conditions can influence the types and amounts of phytochemical produced, leading to a more diverse and potentially more potent extract.
- Improved Stability: Proper extraction methods can help preserve the stability and integrity of phytochemical, preventing degradation and oxidation.
Visual Representation:
By understanding the influence of these factors, researchers and manufacturers can optimise cultivation and extraction practices to obtain Momordica Dioica extracts with the highest possible phytochemical content and antioxidant activity.
Pharmacological and Therapeutic Applications
- Anti-diabetic: Momordica dioica is used to treat diabetes and other metabolic disorders.
- Antioxidant: Momordica dioica has antioxidant properties.
- Anti-inflammatory: Momordica dioica is used to treat inflammation caused by snake bites, lizards, and elephantiasis.
- Antimicrobial: Momordica dioica has antibacterial properties.
- Hepatoprotective: Momordica dioica is used to protect the liver.
- Analgesic: Momordica dioica has analgesic properties.
- Anti-hypertensive: Momordica dioica is used to treat hypertension.
- Diuretic: Momordica dioica has diuretic properties.
- Laxative: Momordica dioica has laxative properties.
- Anti-asthmatic: Momordica dioica is used to treat asthma.
- Antipyretic: Momordica dioica is used to treat fever.
- Anthelminthic: Momordica dioica has antihelminthic properties.
- Spermicidal: Momordica dioica has spermicidal properties.
Potential Therapeutic Uses:
1. Anti-diabetic: Studies have shown that Momordica dioica may help regulate blood sugar levels, potentially making it useful in the management of diabetes.
2. Anti-inflammatory: The plant contains compounds with anti-inflammatory properties, which could be beneficial in treating conditions like arthritis and inflammatory bowel disease.
3. Antioxidant: Momordica dioica is rich in antioxidants, which can help protect cells from damage and may play a role in preventing chronic diseases.
4. Anti-cancer: Some studies suggest that certain compounds in Momordica dioica may have anti-cancer properties, although more research is needed in this area.
5. Antimicrobial: The plant has shown antimicrobial activity against certain bacteria and fungi, which could be useful in developing new antibiotics.
6. Standardisation of extracts: The composition of Momordica dioica can vary depending on growing conditions and harvesting methods, making it difficult to standardise extracts for use in medications.
7. Toxicity: While generally considered safe, high doses of Momordica dioica can cause side effects like diarrhoea and low blood sugar.
8. Drug interactions: More research is needed to understand how Momordica dioica may interact with other medications.
CONCLUSION
The phytochemical profile of Momordica dioica is rich in various bioactive compounds, including flavonoids, triterpenoids, alkaloids, and saponins. These compounds contribute to the plant's antioxidant properties, as evidenced by its ability to scavenge free radicals and reduce oxidative stress. The antioxidant activity of Momordica dioica suggests its potential as a natural remedy for various health conditions associated with oxidative stress, such as cardiovascular disease, diabetes, and cancer. However, further research is needed to fully understand the mechanisms underlying its antioxidant effects and to evaluate its safety and efficacy in humans.
REFERENCE
- Walters TW, Decker DS, Notes on economic plants, Balsam pear (Momordica charantia, Cucurbitaceae),Econ Bot, 42, 1988, 286-28
Bharathi LK, Munshi AD, Chandrashekaran S, Behera TK, Das AB, John KJ. Cytotaxonomical analysis of Momordica L. (Cucurbitaceae) species of Indian occurrence. Journal of Genetics. 2011;90
- Abuajah CI, Ogbonna AC, Osuji CM. Functional components and medicinal properties of food: a review. J Food Sci Technol. 2015;52:2522–2529. doi: 10.1007/s13197-014-1396-5. [DOI] [PMC free article
- Rodríguez-Daza M.C., Pulido-Mateos E.C., Lupien-Meilleur J., Guyonnet D., Desjardins Y., Roy D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021;8:689456. doi: 10.3389/fnut.2021.689456.
- Beltrán D., Romo-Vaquero M., Espín J.C., Tomás-Barberán F.A., Selma M.V. Ellagibacter Isourolithinifaciens Gen. Nov., Sp. Nov., a New Member of the Family Eggerthellaceae, Isolated from Human Gut. Int. J. Syst. Evol. Microbiol. 2018;68:1707–1712. doi: 10.1099/ijsem.0.002735
- Rowland I., Faughnan M., Honey L., Wähälä K., Williamson G., Cassidy A. Bioavailability of Phyto-Oestrogens. Br. J. Nutr. 2003;89:S45–S58. doi: 10.1079/BJN2002796.
- Chang CC, Yang MH, Wen HM and Chern JC, “Estimation of total flavonoid content in propolis by two complementary colorimetric methods”, J Food Drug Anal, 10(3): 178-182, 2002.
- Slinkard K and Singleton VL, “Total Phenol Analysis: Automation and Comparison with Manual Methods”, Am J Enos Vitic, 28: 49-55, 1977
- Das K, Tiwari RKS, Shrivastava DK. Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. Journal of Medicinal Plants Research 2010; 4(2): 104-111.
- Nikhil SB, Dambe PA, Ghongade DB, Goupale DC. Hydroalcoholic extraction of Mangifera indica (leaves) by Soxhletion. International Journal of Pharmaceutical Sciences 2010; 2 (1): 30-32.
- Ncube NS, Afolayan AJ, Okoh AI. Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. African Journal of Biotechnology 2008; 7 (12): 1797-1806.
- 12.Remington JP. Remington: The science and practice of pharmacy, 21st edition, Lippincott Williams & Wilkins, 773-774.
- Plants. International centre for science and high technology, Trieste, 2008, 21-25.
- Evans.W.C, “Treaseand Evans Pharmacognosy”, Harcourt Brace and company. Asia pvt. Ltd.Singapore, 1997. [7] Wagner.H, „Pharmazeutische Biologic”, 5 th Edition, AUFI.15 BN 3-437-20 498-X, 1993. [8] Yasuma. A and Ichikawa. “Ninhydrin-Schiff and alloxan- Schiff staining. A new histochemical staining methods for proteins”, J. Lab clin Med, 1953, 41:296-299. [9] Kokate,C.K, “Practical pharmacognosy” 4 th edition, Vallabh Prakashan Publication, New Delhi, India, 1999.
- Aberoumand A. Screening of less known two food plants for comparison of nutrient contents: Iranian and Indian vegetables. Functional Foods in Health and Disease. 2011;10:416–423.
- Singh D, Bahadur V, Singh DB, Ghosh G. Spine gourd (Momordica dioica): an under-utilised vegetable with high nutritional and medicinal values. ISHS Acta Horticulturae. 2009;809:241–248.
- Ali A, Deokule SS. Comparison of phenolic compounds of some edible plants of Iran and India. Pakistan Journal of Nutrition. 2008;8:26–31.
- Maharana T, Tripathy P. Agrotechniques of Momordica dioica growing spine gourd in pots. Indian Horticulture. 1996:16–17.
- Ram D, Banerjee MK, Pandey S, Srivastava U. Collection and evaluation of Kartoli (Momordica dioica Roxb. Ex. Willd) Indian Journal of Plant Genetic Resource. 2001;14:114–116.
- Bharathi LK, Naik G, Singh HS, Dora DK, Peter KV. Spine gourd. In: Peter KV, editor. Underutilised and Underexploited Horticultural Crops. New Delhi, India: New India Publishing; 2007. pp. 289–295.
- National Plant Data Center. NRCS, USDA, Baton Rouge, La, USA, http://plants.usda.gov/
- Tirmizi SA, Wattoo MHS, Mazhar M, Wattoo FH, Memon AN, Iqbal J. Analytical investigation of chromium and zinc in sweet, sour and bitter tasting fruits, vegetables and medicinal plants. Quimica Nova. 2007;30(7):1573–1577.
- Ghosh A. Mechanism of monocarpic senescence of Momordica dioica: source-sink regulation by reproductive organs. Pakistan Journal of Scientific and Industrial Research. 2005;48(1):55–56.
- Bhuiya MRH, Habib AKMA, Rashid MM. Content and loss of vitamin C in vegetables during storage and cooking. Bangladesh Horticulture. 1977;5:1–6
- Rao MK. Flora of Maharashtra State, Dicotyledons. 2001;2
- Kumara KN, Bulugahapitiya VP. A preliminary chemical study on secondary metabolites present in fruits of Momordica dioica (Thumbakariwila). Proceedings of the 2nd Academic Sessions; 2004; p. p. 96.
- Jian CC, Ming HC, Rui LN, Cordel GA, Qiuz SX. Cucurbitacins and cucurbitane glycosides: structures and biological activities. Natural Product Reports. 2005;22(3):386–399. doi: 10.1039/b418841c. [DOI]
- Ali M, Srivastava V. Characterisation of Phytoconstituents of the Fruits of Momordica dioica. Indian Journal of Pharmaceutical Sciences. 1998;60(5):287–289.
- Sadyojatha AM, Vaidya VP. Chemical constituents of the roots of momordica dioica roxb. Indian Drugs. 1996;33(9):473–475.
- Ghosh BN, Dasgupta B, Sircar PK. Purification of lectin from a tropical plant Momordica dioica Roxb. Indian Journal of Experimental Biology. 1981;19(3):253–255.
- Joshi SR, Vasantha K, Robb JS. An unusual anti-H lectin inhibited by milk from individuals with the Bombay phenotype. Immunohematology. 2005;21(1):1–4.
- Luo L, Li Z, Zhang Y, Huang R. Triterpenes and steroidal compounds from Momordica dioica. Yaoxue Xuebao. 1998;33(11):839–842
- Harborne.J.B, “Phytochemical Methods: A Guide To Modern Techniques of Plant Analysis.” 2nd Edition, Chapmann and Hall Publishers, London, 1998. [
- Finar,I.L, “Stereo Chemistry and the Chemistry of Natural Products” Longman, VOL.2, 1986.
- Rasch. E. and H.Swift, “Micro Photometric Analysis of the Cytochemical million eaction”, J.Histochem.Cytochem, 1960, 8:4-17
- Sattya naraya Talukdar and Mohammad Nazir Hossain (2014):phytochemical, phytotherapeutical and pharmacological study of Momordica dioica, Evidence-based complementary and Alternative Medicine, 11pages
- Adisakwattana S, Roengsamran S, Hsu WH, Yibchoknnun S. Mechanisms of antihyperglycemic effect of p-methoxycinnamic acid in normal and Streptozotozin-induced diabetic rats. Life Sci. 2005;78:406–12. doi: 10.1016/j.lfs.2005.04.073.
- Kanchana G, Shyni WJ, Malini P, Rajadurai M. Effect of sinapic acid on antiperoxidative and antioxidant potential in normal and streptozotocin-induced diabetes in wistar rats. Int J Pharm Clin Res. 2011;3:5–9.
- Ahmed RG. The physiological and biochemical effects of diabetes on the balance between oxidative stress and antioxidant defense system. Med J Islam World Acad Sci. 2005;15:31–42.
- Kim KS, Lee S. Antioxidant activities of the extracts from the herbs of Artemisis apiacea. J Ethnopharmacol.2003;85:69–72. doi: 10.1016/s0378-8741(02)00338-0.
- Kawamura M, Heinecke JW, Chait A. Pathophysiological concentrations of glucose promote oxidative modification of low density lipoprotein by a superoxide-dependent pathway. J Clin Invet. 1994;94:771–8. doi: 10.1172/JCI117396.
- Rosen, H.; Klebanoff, S.J.; Wang, Y.; Brot, N.; Heinecke, J.W.; Fu, X. Methionine oxidation contributes to bacterial killing by the myeloperoxidase system of neutrophils. Proc. Natl. Acad. Sci. USA 2009, 106, 18686–18691. [Google Scholar] [CrossRef] [PubMed]
- Bashan, N.; Kovsan, J.; Kachko, I.; Ovadia, H.; Rudich, A. Positive and negative regulation of insulin signalling by reactive oxygen and nitrogen species. Physiol. Rev. 2009, 89, 27–71. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar]
- Schwab, L.; Goroncy, L.; Palaniyandi, S.; Gautam, S.; Triantafyllopoulou, A.; Mocsai, A.; Reichardt, W.; Karlsson, F.J.; Radhakrishnan, S.V.; Hanke, K.; et al. Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance graft-versus-host disease via tissue damage. Nat. Med. 2014, 20, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Kiley, P.J.; Storz, G. Exploiting thiol modifications. PLoS Biol. 2004, 2, e400. [Google Scholar] [CrossRef] [PubMed]
- Reddie, K.G.; Carroll, K.S. Expanding the functional diversity of proteins through cysteine oxidation. Curr. Opin. Chem. Biol. 2008, 12, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A. Proteomics: A new approach to investigate oxidative stress in Alzheimer’s disease brain. Brain Res. 2004, 1000, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Castegna, A.; Aksenov, M.; Thongboonkerd, V.; Klein, J.B.; Pierce, W.M.; Booze, R.; Markesbery, W.R.; Butterfield, D.A. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part II: Dihydropyrimidinase-related protein 2, ?-enolase and heat shock cognate 71. J. Neurochem. 2002, 82, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Castegna, A.; Thongboonkerd, V.; Klein, J.B.; Lynn, B.; Markesbery, W.R.; Butterfield, D.A. Proteomic identification of nitrated proteins in Alzheimer’s disease brain. J. Neurochem. 2003, 85, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.; Giraudo, S.; Corpillo, D.; Bergamasco, B.; Lopiano, L.; Fasano, M. Proteome analysis of human substantia nigra in Parkinson’s disease. Proteomics 2004, 4, 3943–3952. [Google Scholar] [CrossRef] [PubMed]
- Castegna, A.; Aksenov, M.; Aksenova, M.; Thongboonkerd, V.; Klein, J.B.; Pierce, W.M.; Booze, R.; Markesbery, W.R.; Butterfield, D.A. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part I: Creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic. Biol. Med. 2002, 33, 562–571. [Google Scholar] [CrossRef]
- Mattson, M.P.; Gary, D.S.; Chan, S.L.; Duan, W. Perturbed endoplasmic reticulum function, synaptic apoptosis and the pathogenesis of Alzheimer’s disease. Biochem. Soc. Symp. 2001, 67, 157–162. [Google Scholar] [CrossRef]
- Glaser, C.B.; Yamin, G.; Uversky, V.N.; Fink, A.L. Methionine oxidation, ?-synuclein and Parkinson’s disease. BBA-Proteins Proteo. 2005, 1703, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, J.; Arner, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.; Anderson, M.E. Glutathione. Ann. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R. Tissue-specific functions of individual glutathione peroxidase. Free Rad. Biol. Med. 1999, 27, 951–965. [Google Scholar] [CrossRef]
- Arnér, E.S.; Holmgren, A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000, 267, 6102–6109. [Google Scholar] [CrossRef] [PubMed]
- Bannister, J.V.; Bannister, W.H.; Rotilio, G. Aspects of the structure, function, and applications of superoxide dismutas. CRC Crit. Rev. Biochem. 1987, 22, 111–180. [Google Scholar] [CrossRef]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Rad. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Rice, M.E. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000, 23, 209–216. [Google Scholar] [CrossRef]
- Halliwell, B. Role of free radicals in the neurodegenerative diseases. Drug. Aging 2001, 18, 685–716. [Google Scholar] [CrossRef]
- McCay, P.B. Vitamin E: Interactions with free radicals and ascorbate. Ann. Rev. Nutr. 1985, 5, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Tan, D.X.; Chen, L.; Poeggeler, B.; Manchester, L.; Reiter, R. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr. J. 1993, 1, 57–60. [Google Scholar]
- Reiter, R.J.; Calvo, J.R.; Karbownik, M.; Qi, W.; Tan, D.X. Melatonin and its relation to the immune system and inflammation. Ann. N. Y. Acad. Sci. 2000, 917, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Acuna-Castroviejo, D.; Burkhardt, S.; Karbownik, M. Melatonin: Mechanisms and actions as an antioxidant. Curr. Top. Biophys. 2000, 24, 171–184. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Karbownik, M.; Calvo, J.R. Significance of melatonin in antioxidative defense system: Reactions and products. Neurosignals 2000, 9, 137–159. [Google Scholar] [CrossRef]
- Turunen, M.; Olsson, J.; Dallner, G. Metabolism and function of coenzyme Q. BBA-Biomembranes 2004, 1660, 171–199. [Google Scholar] [CrossRef] [PubMed]
- Hornykiewicz, O. Biochemical aspects of Parkinson’s disease. Neurology 1998, 51, S2–S9. [Google Scholar] [CrossRef] [PubMed]
- LeWitt, P.A. Levodopa for the treatment of Parkinson’s disease. N. Engl. J. Med. 2008, 359, 2468–2476. [Google Scholar] [CrossRef] [PubMed]
- Mena, M.A.; Casarejos, M.J.; Solano, R.M.; de Yebenes, J.G. Half a century of L-DOPA. Curr. Top. Med. Chem. 2009, 9, 880–893. [Google Scholar] [PubMed]
- Group EAIS. Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovas. Dis. (Basel, Switzerland) 2003, 15, 222. [Google Scholar]
- Amemiya, S.; Kamiya, T.; Nito, C.; Inaba, T.; Kato, K.; Ueda, M.; Shimazaki, K.; Katayama, Y. Anti-apoptotic and neuroprotective effects of edaravone following transient focal ischemia in rats. Eur. J. Pharmacol. 2005, 516, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, K.; Fujii, K.; Kamouchi, M.; Nakane, H.; Arihiro, S.; Okada, Y.; Ibayashi, S.; Iida, M. Free radical scavenger, edaravone, in stroke with internal carotid artery occlusion. J. Neural. Sci. 2004, 221, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Yuki, S.; Kogure, K. Strong attenuation of ischemic and postischemic brain edema in rats by a novel free radical scavenger. Stroke 1988, 19, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yuki, S.; Watanabe, T.; Mitsuka, M.; Saito, K.I.; Kogure, K. Delayed neuronal death prevented by inhibition of increased hydroxyl radical formation in a transient cerebral ischemia. Brain Res. 1997, 762, 240–242. [Google Scholar] [CrossRef]


 Bhende Kailas*
Bhende Kailas*


 10.5281/zenodo.14616593
10.5281/zenodo.14616593