This study investigates the effect of milk and water kefir granules on sugar solution, focusing on their fermentation characteristics and potential applications in the production of functional beverages. Kefir grains, which are symbiotic cultures of bacteria and yeasts (SCOBY), were introduced into sugar solutions to evaluate their fermentation processes, including pH change, sugar consumption, and the production of lactic acid, alcohol, and other metabolites. The two types of kefir granules—milk and water—were compared to determine any differences in fermentation rate, microbial diversity, and the final composition of the fermented solution. Results indicate that both milk and water kefir grains were capable of fermenting sugar solutions, with milk kefir demonstrating a more complex microbial profile and a greater production of lactic acid, while water kefir showed higher ethanol content and a faster fermentation rate. The findings suggest that both kefir types can be used for producing probiotic-rich beverages, with the milk kefir being more suited for dairy-based functional drinks, while water kefir may offer a suitable alternative for non-dairy and vegan applications. The study also explores the broader implications of kefir fermentation in enhancing the nutritional and health-promoting properties of sugar-based substrates.
Milk kefir, water kefir, sugar solution, fermentation, probiotics, lactic acid, ethanol, functional beverages
Effect of Milk and Water Kefir Granules in Sugar Solution
Kefir is a traditional fermented beverage that has been consumed for centuries, primarily known for its probiotic properties and health benefits. It is typically made by fermenting milk or sugar water with specific symbiotic cultures of bacteria and yeast (SCOBY). There are two main types of kefir: milk kefir and water kefir. Both types are produced through the fermentation of a sugar-rich solution, but they differ in the medium used and the microbial communities involved.
- Milk Kefir: This is made by fermenting milk (cow, goat, or other animal milk) with milk kefir grains, which consist of a combination of bacteria and yeast.
- Water Kefir: This is produced by fermenting a sugar solution (typically water with added sugar) using water kefir grains, which are a different mix of bacteria and yeast than those used in milk kefir.
The granules (or grains) in both milk and water kefir are composed of polysaccharides, proteins, and various microorganisms. These microorganisms—lactic acid bacteria, yeasts, and other microbes—carry out the fermentation process, which converts sugars into various end-products, including alcohol, organic acids, and carbon dioxide. This fermentation not only preserves the sugar solution but also imparts various health benefits, including improved digestion and gut health. The focus of this research topic is to explore the effect of milk and water kefir granules in sugar solutions. Specifically, it examines how these kefir granules impact the fermentation process in a sugar solution, including changes in:
- Fermentation Rate: The time it takes for the sugar solution to ferment into kefir.
- Microbial Activity: How the microbial population in the granules influences fermentation dynamics, including microbial growth, metabolic activity, and the production of metabolites like lactic acid and alcohol.
- Chemical Changes: The transformation of sugars into organic acids, alcohol, and other compounds.
- Nutritional Changes: The shift in the nutritional profile of the sugar solution, such as reduced sugar content and increased levels of beneficial compounds like probiotics.
- Flavor Development: The changes in flavor due to the microbial activity, which can range from tart and tangy (due to lactic acid) to slightly alcoholic or effervescent (due to yeast fermentation).
Understanding how milk and water kefir granules behave in a sugar solution is crucial for optimizing kefir production, both in terms of health benefits and taste. It also contributes to a broader understanding of fermentation science, particularly how different kefir grains interact with varying sugar sources and environmental conditions.
This topic is of interest to researchers in microbiology, food science, and nutrition, and could have implications for the food and beverage industry, where kefir-based products are becoming increasingly popular as functional foods.
MATERIALS AND METHODS
PREPARATION OF WATER KEFIR
Ingredients:
- 2 Tablespoons hydrated Water Kefir Grains
- 1?4 cup sugar – organic preferred
- 1 quart of chlorine free water
Requirements:
- Glass Jar
- Mesh strainer
- Cloth with rubber band
- Wooden or plastic spoon and jars

Fig.3.1 WATER KEFIR SAMPLE
Preparation of primary culture(water kefir)
- 100g of sugar was added to the jar.
- Then 500ml water was added to the jar.
- The mixture was stirred thoroughly until the sugar was dissolved.
- 20g of kefir grains was added to the sugar water. Covered with a plastic lid. Kept aside for 24 hours. The kefir has fermented as it smelled tangy. [10]
- A wide mouthed glass container was placed under the strainer , the finished kefir was poured into the strainer, and stirred with a spoon to gently force kefir through the strainer. The grains were separated and collected into a sterile glass container.
- The grains were stored in the fridge.

Fig. 3.2 WATER KEFIR PRIMARY CULTURE
PREPARATION OF MILK KEFIR
Ingredients:
- 1 Tbsp kefir grains
- 4 cups whole cow’s milk 960 ml
Requirements:
- Large glass jug (at least 5 cup capacity)
- Wooden or plastic spoon
- Paper towels
- Plastic mesh strainer
- Jar for storing finished kefir

Fig.3.3 MILK KEFIR SAMPLE
Preparation of primary culture (Milk Kefir)
- Initially glass jugs, spoons were steriled 20g of the kefir grains and 500ml of whole milk to a large glass jug was added .
- The jug was covered with plastic lid ,to prevent any bugs or dust from getting in. Fermentation set in a warm, dark spot for about 24 hours[11].
- The kefir has fermented as it was slightly thickened and smelled tangy.
- The kefir has separated into yellowish watery-looking whey.
- A wide mouthed glass container was placed under the strainer , the finished kefir was poured into the strainer and stirred with a spoon to gently force kefir through the strainer .
- The grains were separated and collected into a sterile glass container.
The grains were stored in the fridge

Fig.3.4 MILK KEFIR PRIMARY CULTURE
ESTIMATION OF SUGAR BY UV-VISIBLE SPECTROSCOPY
INGREDIENTS :
- Sulphur free sugar
- Sodium hydroxide
- Sodium potassium tatarate
- Distilled water
- Ethanol

REQUIREMENTS
- UV-Visible spectrophotometer
- Volumetric flask
- Pipette
- Beaker
UV –VISIBLE SPECTROPHOTOMETER
PREPARATION OF SOLUTIONS
Glucose solution (100mg %) : 100 mg of standard sulphur free sugar is dissolved in 100ml of distilled water.
2N NaOH : 8g of sodium hydroxide pellets is dissolved in 100 ml of distilled water to form 2N NaOH.
PREPARATION OF DNSA SOLUTION :
- 1g of DNSA (di-nitro salicylic acid) is dissolved in 20 ml of 2N NAOH.
- 50 ml distilled water was added into the flask .
- 30g of Rochelle’s salt was added and was dissolved.
- Volume was madeup to 100 ml with distilled water .It was then filtered in and stored amber coloured bottle.
PREPARATION OF WATER KEFIR SAMPLE:
- 100mg of sugar was added to the jar.
- Then 100ml water was added to the jar.
- The mixture was stirred thoroughly until the sugar was dissolved.
- 300mg of kefir grains was added to the sugar water. Covered with a plastic lid. Kept aside for 24 hours
- The kefir has fermented smelled tangy. [10]
- A wide mouthed glass container was placed under the strainer , the finished kefir was poured into the strainer, and stirred with a spoon to gently force kefir through the strainer .
- The grains were separated and collected into a sterile glass container.
- The grains were stored in the fridge .
PROCEDURE FOR WATER KEFIR :
- 0,2,4,6,8ml of above sample solution was pippetted out into each 25 ml volumetric flask.
- 3 ml of 2N NaOH was added to all flasks.
- 3 ml DNSA solution was added to all flasks.
- The flasks were made up to 25ml .
- Flasks were kept in boiling water for 15 minutes.
- The flasks were kept under the running water and the absorbance was measured at 540 nm.
PREPARATION OF MILK KEFIR SAMPLE :
- 300mg of the kefir grains and 100ml of whole milk to a large glass jar was added .
- The jug was covered with plastic lid ,to prevent any bugs or dust from getting in. Fermentation set in a warm, dark spot for about 24 hours[11].
- The kefir has fermented as it was slightly thickened and it smelled fermented.
- The kefir has separated into yellowish watery-looking whey.
- A wide mouthed glass container was placed under the strainer , the finished kefir was poured into the strainer, and stirred with a spoon to gently force kefir through the strainer .
- The grains were separated and collected into a sterile glass container.
- The grains were stored in the fridge.
PROCEDURE FOR MILK KEFIR
- 0, 2,4,6,8ml of above sample solution was pippetted out into 25 ml volumetric flask.
- Add 3 ml of ethanol to all flasks.
- The flasks were made up to 25 ml using water.
- Stored in the fridge for 1 hour.
- The flasks were brought to normal temperature and the absorbance is measured at 208 nm.
RESULTS AND DISCUSSIONS:
Milk and Water kefir beverages were prepared. The absorbance of the prepared drinks were estimated and compared with the standard sugar water and milk using UV-VISIBLE SPECTROPHOTOMETER. We have observed reduction in the sugar concentration of kefir fermented beverages .Kefir has a tangy flavor and a consistency similar to drinkable yogurt. Due to the fermentation process, kefir tasted slightly carbonated. We have observed the biomass of kefir grains. Initially we have taken 20g of water and milk kefir grains. For 1st culture of water kefir the grains weight increased gradually to 28g for 2nd culture it increased up to 31g & for 3rd culture it increased to 34g. Later the mass of the kefir grains were decreased. Same as for milk kefir, 20g lo kefir grains were taken. For 1st culture it increases upto 26g for 2nd culture it increases up to 29g & for 3rd culture it increases up to 30g after the 3rd culture the weight of the kefir grains were decreased.
ESTIMATION OF ABSORBANCE OF SUGAR CONCENTRATION IN WATER KEFIR
Standard
Table 5.1 Results of absorbance of standard
|
s.no
|
Concentration
(?g/ml)
|
Absorbance
( nm) (standard)
|
|
1.
|
0
|
0
|
|
2.
|
80
|
0.220
|
|
3.
|
160
|
0.426
|
|
4.
|
240
|
0.650
|
|
5.
|
320
|
0.854
|

Fig no.4.1 Calibration curve for standard.
Day -1
Table 5.2 Results of absorbance of water kefir on day -1
|
s.no
|
Concentration
(?g/ml)
|
Absorbance (nm) (standard)
|
Absorbance (nm) (sample)
|
|
1.
|
0
|
0
|
0
|
|
2.
|
80
|
0.220
|
0.220
|
|
3.
|
160
|
0.426
|
0.421
|
|
4.
|
240
|
0.650
|
0.622
|
|
5.
|
320
|
0.854
|
0.849
|
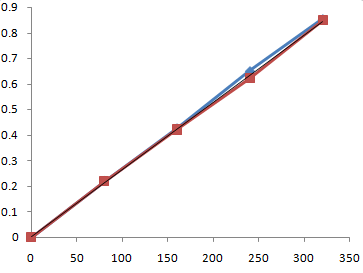
Fig no.4.2 Calibration curve for standard vs sample day 1
Day -2
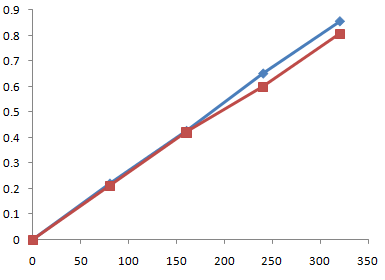
Fig no.4.3 Calibration curve for standard vs sample day 2
Table 5.3 Results of absorbance of water kefir only day-2
|
s.no
|
Concentration
(?g/ml)
|
Absorbance in nm (standard)
|
Absorbance (nm) (sample)
|
|
1.
|
0
|
0
|
0
|
|
2.
|
80
|
0.220
|
0.150
|
|
3.
|
160
|
0.426
|
0.353
|
|
4.
|
240
|
0.650
|
0.499
|
|
5.
|
320
|
0.854
|
0.687
|
Day -3
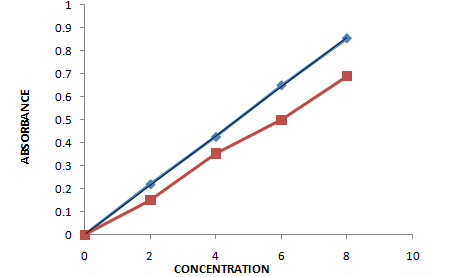
Fig no.4.3 Calibration curve for standard vs sample day 3
Table 5.3 Results of absorbance of water kefir only day-3
|
s.no
|
Concentration
(?g/ml)
|
Absorbance ( nm) (standard)
|
|
1.
|
0
|
0
|
|
2.
|
80
|
0.195
|
|
3.
|
160
|
0.369
|
|
4.
|
240
|
0.561
|
|
5.
|
320
|
0.722
|
ESTIMATION OF ABSORBANCE OF SUGAR CONCENTRATION IN MILK KEFIR
Standard
Table 5.5 Results of absorbance of standard lactose

Fig no.4.4 Calibration curve for milk standard
Day -1
Table 5.6 Results of absorbance of milk kefir on day-1

Fig no.4.5 Calibration curve for milk standard vs sample day1
Day -2
Table 5.7 Results of absorbance of milk kefir on day -2
|
s.no
|
Concentration
(?g/ml)
|
Absorbance in nm (standard)
|
Absorbance (nm) (sample)
|
|
1.
|
0
|
0
|
0
|
|
2.
|
80
|
0.195
|
0.174
|
|
3.
|
160
|
0.369
|
0.344
|
|
4.
|
240
|
0.561
|
0.505
|
|
5.
|
360
|
0.722
|
0.699
|

Fig no.4.6 Calibration curve for milk standard vs sample day2
Day -3
Table 5.7 Results of absorbance of milk kefir on day-3

Fig no.4.4 Calibration curve for milk standard vs sample day3
From the above graphs we can conclude that fermented kefir beverages have shown that there is reduction in sugar concentration when compared with standard.
SUMMARY AND CONCLUSION
Kefir grains have shown the ability to reduce sugar concentration during fermentation, with studies demonstrating a significant decrease in sugar content, such as a reduction of approximately 40?ter 96 hours of fermentation. The conversion of sucrose to glucose and fructose during fermentation contributes to this reduction, with variations observed in sugar content when kefir grains interact with different substrates like organic brown sugar The conversion of sucrose to simpler sugars like glucose and fructose, along with the interaction between kefir grains and various substrates, influences the sugar content in the final product. The fermentation process occurs when milk is combined with kefir grains and left to ferment at room temperature for several days. During this process, the kefir grains break down the lactose in the milk, creating lactic acid, alcohol, and acetic acid. The water kefir grains utilize the sugar in the solution to produce lactic acid, carbon dioxide, and a small amount of ethanol. This process involves the breakdown of sucrose into simpler compounds like glucose and fructose by the microorganisms present in the grains.
REFERENCE
- Abraham, B. P. and Quigley E. M. A probiotics for ulcerative colitis: the culture wars continue. Digestive Diseases and Sciences, 2018, vol. 63, pp. 1678-1680.
- Abraham, A. G. and De Antoni G. L. Characterization of kefir grains in cow's milk and in soya milk. Journal of Dairy Research, 1999, vol. 66, no. 2, pp. 327–333.
- Ahmed, Z., Wang Y., Ahmad A., Khan S. T., Nisa M., Ahmad H. and Afreen A. Kefir and health:a contemporary perspective. Critical reviews in food science and nutrition, 2013, vol. 53, no.5, pp.422-434.
- Akin, M. S. Effects of inulin and different sugar levels on viability of probiotic bacteria and thephysical and sensory characteristics of probiotic fermented ice-cream. Milchwissenschaft, 2005,vol. 60, no. 3, pp. 297-301.
- Alm, L. Effect of fermentation on lactose, glucose, and galactose content in milk and suitability offermented milk products for lactose 2021, vol. 12, no. 2, pp. 355.
- Alter, D. A. and Tu L. V. Socioeconomic status and cardiovascular disease: universal inequities and the challenges that lie ahead. Cardiovascular reviews& reports, 2000, vol. 21no. 12, pp. 655-660.
- Anyanwu, S. N. Temporal trends in breast cancer presentation in the third world. Journal ofExperimental & Clinical Cancer Research, 2008, vol. 7, no. 1, pp. 17.
- Armitrage, J.O. and Longo D.L. Malignancies of lymphoid cells. Harrison’s Online: Harrison’sPrinciples of Internal Medicine. 16thEd. New York, NY: McGraw-Hill; 2005.
- Arslan, SA review: chemical, microbiological and nutritional characteristics of kefir. CyTA-Journal of Food, 2015, vol. 13, no. 3, pp. 340-345.
- Beshkova, D. M., Simova E. D., Simov Z. I., Frengova G. I. and Spasov Z. N. Pure cultures formaking kefir. Food Microbiology, 2002, vol. 19, no. 5, pp. 537–544.
- BourrieB. C., Willing B. P. and Cotter P. D. (2016). The microbiotaand health promotingcharacteristics of the fermented beverage kefir. Frontiersin microbiology, 7, 647. Responsive muscle cells. Cytotechnology, 2016, vol. 40, no. 1-3, pp 107-116.
- Burks, A. W., Tang M., Sicherer S., Muraro A., Eigenmann P. A., Ebisawa M. and Hourihane J.ICON: food allergy. Journal of Allergy and Clinical Immunology, 2012, vol. 129, no. 4, pp. 906-920.
- Chen, C., Chan H.M. and Kubow S. Kefir extracts suppress in vitro proliferation of estrogen-dependent human breast cancer cells but not normal mammary epithelial cells. Journal of MedicinalFood, 2007, vol. 10, no. 3, pp. 416 – 422.
- De Vrese, M. and Marteau, P.R. Probiotics and Prebiotics: effects on diarrhea. The Journal ofNutrition, 2007, vol. 137, no. 3, pp. 803S–811S.
- Farag, M. A., Jomaa, S. A. and El-Wahed A. A. The Many Faces of Kefir Fermented DairyProducts: Quality Characteristics, Flavour Chemistry, Nutritional Value, Health Benefits, andSafety. Nutrients, 2020, vol. 12, no. 2, pp. 346.
- Foster, P. J., Dunn, E. A., Karl K. E., Snir J. A., Nycz C. M., Harvey A. J. and Pettis R. J. Cellularmagnetic resonance imaging: in vivo imaging of melanoma cells in lymph nodes ofmice. Neoplasia, 2008, vol. 10, no. 3, pp. 207-216.
- Gao, X. and Li B. Chemical and microbiological characteristics of kefir grains and their fermenteddairy products: A review. Cogent Food & Agriculture, 2016, vol. 2, no. 1, pp. 1272152.
- Gasbarrini, G., Bonvicini, F. and Gramenzi, A. Probiotics history. Journal of clinicalGastroenterology, 2016, vol. 50, pp. 116-119.
- Gaware, V., Kotade, K., Dolas, R., Dhamak, K., Somwanshi, S., Nikam, V. and Kashid, V. Themagic of kefir: a review. Pharmacology online, 2011, vol. 1, pp. 376-386.
- Gloster, Jr H. M. and Neal, K. Skin cancer in skin of color. Journal of the American Academy ofDermatology, 2006, vol. 55, no. 5, pp. 741-760. Gueguen, L. La valeur nutritionnelle minerale du lait de chevre. INRA, 1997, vol. 81, pp. 67–80.
- Guzel-Seydim, Z. B., Seydim, A. C., Greene, A. K. and Bodine, A. B. Determination of organicacids and volatile flavor substances in kefir during fermentation. Journal of Food Composition andAnalysis, 2000, vol. 13, no. 1, pp. 35–43.
- Hertzler, S.R. and Clancy, S.M. Kefir improves lactose digestion and tolerance in adults with lactosemaldigestion. Journal of the American Dietetic Association, 2003, vol. 103, pp. 582 -587.
- Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B. and Calder, P. C. TheInternational Scientific Association for Probiotics and Prebiotics consensus on the scope andappropriate use of the term.
- Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B. and Calder, P. C. The International Scientific Association for Probiotics and Prebiotics consensus on the scope and appropriate use of the term probiotics . Nature Reviews Gastroenterology &Hepatology, 2014, vol. 11, no. 8, pp. 506–514.
- ?rge, E., Timur, S., Zincir, H., Oltuluo?lu, H., Dursun, S. and Gebelikte beslenmenin de?erlendirilmesi. STED, 2005, vol. 14, no. 7, pp. 157-160 (Turkish).
- John, S. M. and Deeseenthum, S. Properties and benefits of kefir-A review.
- Karamanou, M., Protogerou, A., Tsoucalas, G., Androutsos, G. and Poulakou- Rebelakou, E. Milestones in the history of diabetes mellitus: the main contributors. World Journal of Diabetes, 2016, vol. 7, no.1, pp.1–7.
- Kardakova, M. and Ghoddusi, H. Fermented milk kefir: probiotic content and potential health benefits. Research gate, 2018.
- Kehinde, B. A. and Sharma, P. Recently isolated anti diabetic hydrolysates and peptides from multiple food sources: A review. Critical reviews in food science and nutrition, 2020, vol. 60, no. 2, pp. 322-340.
- Kesenkas, H., Dinkci, N., Seckin, K., Kinik, O., Gönc, S., Ergönül, P. G. and Kavas, G. Physicochemical, microbiological and sensory characteristics of soymilk kefir. African Journal of Microbiology Research, 2011, vol. 5, no. 22, pp. 3737–3746.
- Kesenkas, H., Yerlikaya, O. and Ozer, E. A. Functional milk beverage: Kefir. Agro FOOD Industry Hi Tech, 2013, vol. 24, pp. 53–55.
- Kneifel, W. and Mayer, H. K.Vitamin profiles of kefirs made from milks of different species. International Journal of Food Science and Technology, 1991, vol. 26, no. 4, pp. 423–428.
- Kok-Tas, T., Ekinci, F. Y. and Guzel-Seydim, Z. B. Identification of microbial flora in kefir grains produced in Turkey using PCR. International Journal of Dairy Technology, 2012, vol. 65, no. 1, pp. 126–131.
- Kolberg, H. C., Schneeweiss, A., Fehm T. N., Wöckel A., Huober J., Pontones C. and Hartkopf A. D. Update Breast Cancer 2019 Part 3–Current Developments in Early Breast Cancer: Review and Critical Assessment by an International Expert Panel. Geburtshilfe und Frauenheilkunde, 2019, vol. 79, no. 5, pp. 470.
- Koohi, F., Shamlou, R., Eslami, S., Ghojogh, Z. M., Kor, Y. and Rafiemanesh, H. Leukemia in Iran: epidemiology and morphology trends. Asian Pacific Journal of Cancer Prevention, 2015, vol. 16, no. 17, pp. 7759-7763.
- Kurman, J.A., Raši?, J.L., Kroger, M. Encyclopedia of Fermented Fresh Milk Products, an international inventory of fermented milk, cream, buttermilk, whey, and related products. Springer Science & Business Media, 1992.
- Liu, J. R., Wang, S. Y., Chen, M. J., Yueh, P. Y. and Lin, C. W. The anti?allergenic properties of milk kefir and soymilk kefir and their beneficial effects on the intestinal microflora. Journal of the Science of Food and Agriculture, 2006, vol. 86, no. 15, pp. 2527-2533.
- Liut Kevicius, A. and Sarkinas, A. A Studies on the growth conditions and composition of kefir grains as a food and forage biomass. Dairy Science Abstracts, 2004, vol. 66, pp. 903.
- Maalouf, K., Baydoun, E. and Rizk S. Kefir induces cell-cycle arrest and apoptosis in HTLV-1- negative malignant T-lymphocytes. Cancer Management and Research, 2011, vol. 3, pp. 39.
- Magalhães, K. T., Pereira, G. V. D. M., Campos, C. R., Dragone G. and Schwan R. F. Brazilian kefir: structure, microbial communities and chemical composition. Brazilian Journal of Microbiology, 2011, vol. 42, no. 2, pp. 693-702
- Malik, T. F. and Panuganti, K. K. Lactose Intolerance. In: StatPearls. StatPearls Publishing, Treasure Island (FL), 2019.
- Marshall, V. M. and Cole, W. M. Methods for making kefir and fermented milks based on kefir. Journal of Dairy Research, 1985, vol. 52, no.3, pp. 451-456.
- McFarland, L.V. From yaks to yogurt: the history, development, and current use of probiotics. Clin Infect Diseases, 2015, vol. 60, no. 2, pp. 85–90.
- Mei, J., Guo, Q., Wu, Y. and Li Y. Microbial diversity of a camembert-type cheese using freezedried tibetan kefir co culture as starter culture by culture-dependent and culture-independent methods. PLoS ONE, 2014, vol. 9, no. 10, pp. e111648.
- Momenimovahed, Z. and Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer: Targets and Therapy, 2019, vol. 11, pp. 151.


 Rongali Indu*
Rongali Indu*












 10.5281/zenodo.14206456
10.5281/zenodo.14206456