Abstract
CRISPR-Cas9 has emerged as a revolutionary tool in gene editing, enabling precise alterations in the DNA sequence with unprecedented accuracy and efficiency. This technology leverages the natural defense mechanism of bacteria against viruses, allowing researchers to target specific genes for modification, deletion, or insertion. The potential applications of CRISPR-Cas9 are vast, ranging from therapeutic interventions for genetic disorders, cancer treatment, and viral infections to advancements in agriculture and biotechnology. Despite its promise, CRISPR-Cas9 raises significant ethical concerns. Issues such as off-target effects, the long-term safety of edited genomes, and the potential for unintended genetic consequences remain critical hurdles. Additionally, the prospect of human germline editing sparks debate around 'designer babies,' eugenics, and the moral implications of altering the human genome for non-therapeutic purposes. This review explores the latest developments in CRISPR-Cas9 technology, highlighting its transformative applications in various fields of research and medicine. It also examines the ethical challenges and regulatory frameworks needed to navigate this rapidly evolving landscape. By addressing both the potential and the pitfalls of CRISPR-Cas9, this article aims to provide a comprehensive overview of the current state of gene editing technology and its future prospects in shaping the genetic landscape.
Keywords
CRISPR-Cas9, Gene Editing, Genome Modification, Therapeutic Applications, Editing, Biotechnology
Introduction
The field of genetic research has experienced a monumental shift in the past decade, primarily driven by the development and widespread adoption of the CRISPR-Cas9 gene-editing technology. Discovered in 2012 by scientists Jennifer Doudna and Emmanuelle Charpentier, CRISPR-Cas9 has quickly transformed molecular biology, offering an efficient, precise, and cost-effective method for altering the DNA of living organisms. Unlike traditional genetic modification techniques, which are often labour-intensive and imprecise, CRISPR-Cas9 allows for targeted cuts in DNA at specific sites, making it possible to add, remove, or modify genes with unprecedented accuracy [1,2].
The potential applications of CRISPR-Cas9 are vast and span multiple fields, including medicine, agriculture, biotechnology, and environmental science. In the medical field, CRISPR has already shown promise in treating genetic disorders such as sickle cell anemia, cystic fibrosis, and muscular dystrophy by directly correcting mutations at the DNA level. In agriculture, CRISPR is being used to develop crops with enhanced nutritional profiles, disease resistance, and environmental tolerance, thus helping to address food security challenges. The biotechnology industry is also benefiting from CRISPR’s ability to engineer microorganisms for the production of biofuels, pharmaceuticals, and industrial chemicals [3].
However, alongside the excitement surrounding its potential, the CRISPR-Cas9 technology raises several ethical, legal, and social concerns. Perhaps the most controversial application is germline editing, which involves making genetic changes to human embryos or germline cells that would be passed down to future generations. Such applications have sparked heated debates over issues like "designer babies," genetic inequality, and the long-term impact of genetic modifications. Additionally, there are concerns about unintended consequences, off-target effects, and the ecological risks of using CRISPR in wild organisms and ecosystems [4].
This review aims to explore the mechanism of CRISPR-Cas9, its applications in various fields, and the ethical implications of its use in gene editing. It will examine how CRISPR-Cas9 works at the molecular level, its transformative role in modern science, and the potential risks and benefits of its widespread use. As we stand on the brink of a genetic revolution, understanding the technology and its ethical boundaries is essential for guiding its future applications and ensuring it is used responsibly for the benefit of humanity [5].
CRISPR-Cas9 Technology
The CRISPR-Cas9 technology, short for Clustered Regularly Interspaced Short Palindromic Repeats and CRISPR-associated protein 9, is a groundbreaking tool that has revolutionized the field of molecular biology and gene editing. Its development, which earned Jennifer Doudna and Emmanuelle Charpentier the Nobel Prize in Chemistry in 2020, has rapidly advanced genetic research, offering scientists a precise, efficient, and adaptable means of altering DNA in living organisms. CRISPR-Cas9 has expanded the possibilities of genetic engineering across various disciplines, including medicine, agriculture, and biotechnology, providing opportunities for targeted genetic modifications that were previously unachievable with earlier technologies [6].
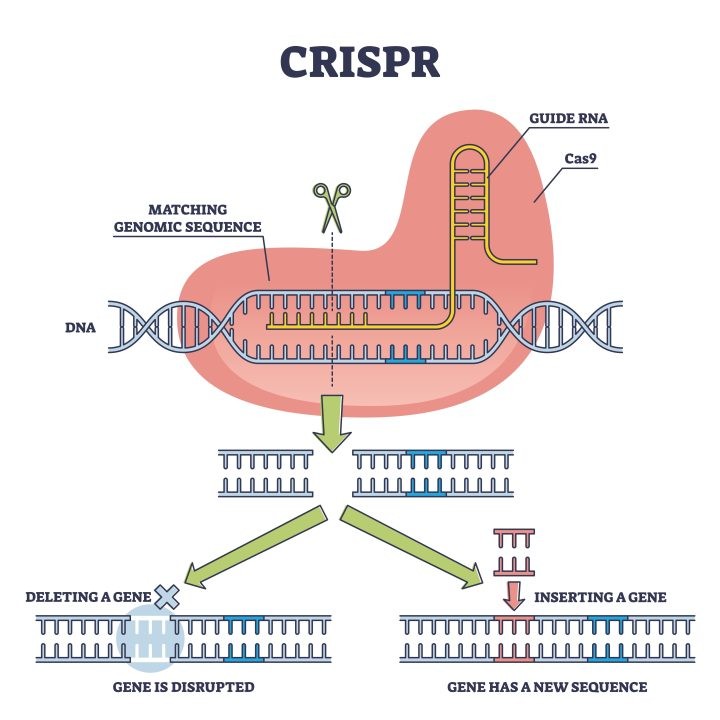
Figure 1: CRISPR-Cas9 Technology
With this method, almost every genomic sequence that is indicated by a brief guide RNA segment can be precisely altered, utilizing a fusion protein of nuclease-deficient Cas9 and effector domain, enabling the clarification of gene function implicated in disease development and progressions, correction of disease-causing mutations, and inactivation of activated oncogenes or activation of deactivated cancer suppressor genes Furthermore, by simultaneously targeting multiple genomic loci in a single experiment, programmable endonuclease technology allows researchers to investigate the function of multiple genes simultaneously. This significantly advances our understanding of pathological processes involving large sets of genes or mutations, like tumor development. CRISPR-based genome-wide screens can be used to rapidly evaluate drug targets and find drug-target or disease-resistance genes, such as new tumor suppressors or oncogenes, by utilizing single-guide RNA (sgRNA) libraries. Therefore, CRISPR–Cas9-mediated genome engineering presents enormous potential for the treatment or even the cure of genetic illnesses, such as sickle cell anemia, cystic fibrosis, Duchenne muscular dystrophy, viral infections, immunological disorders, and cardiovascular diseases, as well as numerous types of cancer and neurodegeneration [7,8].
Mechanism of CRISPR-Cas9
The CRISPR-Cas9 system was originally discovered as a part of the immune system in bacteria and archaea, where it functions as a defense mechanism against viral infections. The system works through a sequence of steps that allow organisms to "remember" and defend against past viral invaders. The discovery of CRISPR-Cas9 as a tool for gene editing was made possible by the realization that its mechanism could be adapted to cut DNA at precise locations in any organism’s genome [9].
1.1. Components of the CRISPR-Cas9 System
CRISPR Sequences:
The CRISPR sequences are short, repetitive DNA sequences found in the genomes of bacteria and archaea. These sequences are interspersed with segments known as spacers, which are derived from viral DNA sequences. The spacers serve as a molecular memory of past viral infections, allowing bacteria to recognize and target the same viral DNA in future encounters.
Cas9 Protein:
Cas9 (CRISPR-associated protein 9) is an endonuclease, meaning it acts as molecular scissors that can cut DNA. Cas9 is guided by RNA molecules to specific locations on the DNA strand, where it induces a double-strand break. The ability of Cas9 to target specific genetic sequences is what makes CRISPR-Cas9 a powerful tool for gene editing.
Guide RNA (gRNA):
The guide RNA is a short RNA sequence designed to be complementary to the target DNA sequence. The gRNA binds to the target DNA and directs the Cas9 protein to the correct location. The specificity of the gRNA allows researchers to target virtually any gene in any organism.
1.2. The Gene Editing Process
The gene editing process using CRISPR-Cas9 involves several critical steps:
Designing the Guide RNA:
To begin, a specific guide RNA (gRNA) is designed to match a particular DNA sequence of interest. This RNA sequence is complementary to the target gene and directs the Cas9 protein to the precise location in the genome.
Cas9 and gRNA Binding:
The guide RNA and the Cas9 protein are introduced into the cell. The gRNA binds to the target DNA sequence, and Cas9 is directed to this site.
DNA Cleavage:
Once at the target site, the Cas9 protein induces a double-strand break in the DNA. This break is critical, as it triggers the cell's natural DNA repair mechanisms.
DNA Repair Mechanisms:
After the DNA is cut, the cell attempts to repair the break. There are two main pathways through which this repair can occur:
Non-Homologous End Joining (NHEJ): This repair process directly ligates the two broken ends of the DNA together. However, it is error-prone and may introduce insertions or deletions (indels), which can disrupt the function of the target gene.
Homology-Directed Repair (HDR): If a DNA template is provided, the cell can repair the break with high precision by incorporating the desired genetic modification. This repair mechanism allows for the insertion of new genetic material or the correction of mutations.
1.3. Precision and Efficiency
The precision of CRISPR-Cas9 lies in the ability of the guide RNA to match the target DNA sequence, ensuring that only the intended gene is edited. While CRISPR is highly efficient, it is not without its limitations. One challenge is the potential for off-target effects, where the Cas9 protein might cut at unintended locations in the genome. Researchers have worked to refine the technology by developing new versions of Cas9 (e.g., Cpf1, Cas12) and guide RNA designs that reduce these off-target effects and enhance the specificity of the editing process [9-13].
Genome Editing:
The term "genome editing technology" refers to a group of technologies that can be used to modify the sequences of cellular DNA at specific genomic sites. These altered sequences are produced by nuclease-mediated site-specific DNA breaks, which are then repaired by DNA repair pathways. Clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein (Cas) nucleases are the most often utilized genome editing-associated nucleases due to their convenience, efficiency, and precision The CRISPR-Cas9 system was quickly shown to be an effective tool for editing eukaryotic genomes when it was described and designed to carry out RNA-guided DNA cleavage at particular places in prokaryotes [9]. Since then, substantial development and global attention have been brought to CRISPR-based genome editing technology.By choosing the best CRISPR-Cas tools, new CRISPR-based tools with expanded targeting ranges,enhanced editing specificity and efficiency, and other unique features have made eukaryotic genome editing easier. By integrating additional effector proteins, this system has been used to enhance the CRISPR-Cas nuclease arsenal and address transcriptional regulation, epigenetic alteration, and live-cell imaging [14].
A new era of precision medicine based on genome editing has begun as a result of the exponential development of genome editing technology, which has fundamentally altered the landscape of biological and medical research.Target DNA can be cut by CRISPR-based nucleases, which then cause double-strand breaks (DSBs) and introduce random mutations by non-homologous end-joining (NHEJ) or homology-directed repair (HDR) for precise editing. Using mice models of several human diseases, the therapeutic potential of CRISPR-based technologies has been explored.Nevertheless, accurate gene correction for in vivo therapeutic value is still difficult, in part because HDR-mediated DNA replacement is not very effective. Post-mitotic cells typically cannot use this tactic because HDR primarily happens during the S/G2 phase of cell division.By combining activity-impaired Cas nucleases with deaminases, also known as base editors, or with reverse transcriptases, also known as prime editors (PEs), precise genome editing tools have been created and are continuously enhanced [15].
General Mechanism of Action:
Three phases comprise the CRISPR-Cas immune response: expression, interference, and adaptability. A complex of Cas proteins comes into contact with a short protospacer-adjacent motif (PAM) during the adaptation process. It then attaches itself to the invasive DNA molecule and breaks two double strands in it. The protospacer, a brief DNA fragment released by invasive phages or plasmids, integrates between two CRISPR array repetitions and functions as a spacer. The expression of the cas genes and the transcription of the CRISPR into a lengthy precursor CRISPR RNA (pre-crRNA) take place during the expression stage. Pre-crRNA is converted into short, mature crRNA by auxiliary factors and cas proteins. During the interference step, the foreign nucleic acid is recognized and its cleavage is mediated by the joint action of Cas proteins and crRNA, therefore protecting the host cells from infection. The expression and interference steps are distinct in each of the CRISPR systems. In type I, hairpin loops create a junction between double-stranded RNA (dsRNA) and single-stranded RNA (ssRNA), which is where Cas6e/Cas6f cut. TransactivatingcrRNAs (tracrRNAs) in the type II system contribute to the formation of dsRNA, which is cleaved by RNase III and Cas9. Hairpin loops are not necessary for type III cleavage, which uses a Cas6 homolog in the direct repeat.Both plasmid and chromosomal DNA include CRISPRs. Within a CRISPR locus, spacer lengths and repeat lengths and sequences are highly conserved; however, they might vary throughout CRISPRs in the same or different genomes [16].

Figure 2: Mechanism of CRISPR-Cas9
Although repetitions usually have lengths of 28–37 nt and each contain a palindromic sequence that can form hairpin structures, CRISPR repeats can vary in length (23–55 nt). Similarly, spacers have a wide range of lengths (21–72 nt), while 32–38 nt is the average. As was already established, the CRISPR system is useful for genomic engineering since a variety of Cas proteins can bind to nucleic acids. Cas 9 is the Cas protein most frequently utilized for control and editing of the genome [27]. Figure 1 shows the molecular mechanism of genome editing mediated by the CRISPR–Cas9 system.The two nuclease domains that make up Cas9 are the RuvC-like nuclease, which divides into RuvC-I, RuvC-II, and RuvC-III subdomains and cleaves the nontarget strand, and the HNH (His–Asn–His) nuclease, which cleaves the target strand of DNA. Two RNAs, the tracrRNA, which hybridizes with the crRNA and is specific to the type II, and the crRNA, which detects the foreign DNA, combine to form a ribonucleoprotein complex with Cas9. The tracrRNA and crRNA can be combined to form a chimeric single-guide RNA (sgRNA) for effective genome editing. sgRNA is made up of two segments: a 20 nt segment that is complementary to the target DNA sequence and a constant segment that serves as a scaffold for Cas9 binding and the 5? end [17].
Applications of CRISPR/CAS-9:
Only a few years after its discovery, the CRISPR/Cas-9 genome editing tool has already been investigated for a variety of uses and significantly impacted the globe in a number of fields, including biotechnology, agriculture, and medicine. Researchers anticipate that this technology will continue to evolve in the future to eradicate infectious diseases, create more nutrient-dense crops, and treat and cure illnesses.
The following section discusses some of the recent CRISPR/Cas-9 uses and clinical trials under investigation.
General Application:
Genome editing has been transformed by CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats), which provides accurate, effective, and versatile ways to alter genetic material in a broad range of organisms. The following are some significant genome editing uses for CRISPR:
- Biomedical Research:
Gene Function Studies: Researchers can examine gene function and comprehend biological processes by using CRISPR to knock out genes in cell lines or animal models.
Disease Modeling: CRISPR can produce animal models (such as mice or zebrafish) for diseases including diabetes, cancer, and neurological diseases by introducing particular mutations linked to those ailments.
Therapeutic Development and Discovery: By demonstrating how genes interact with medications and by evaluating their toxicity and efficacy, CRISPR-modified cells can be used to screen possible therapeutic targets.
- Gene Therapy and Treatment of Genetic Disorders:
Correction of Mutations: By modifying the faulty genes in patients' cells, CRISPR has demonstrated promise in repairing mutations that result in hereditary illnesses such muscular dystrophy, sickle cell anemia, and cystic fibrosis.
Vivo and Ex Vivo Methods: CRISPR delivery techniques, like as viral vectors or nanoparticles, can be used to edit cells directly inside the body (in vivo) or outside the body (ex vivo) and subsequently reintroduced to patients for specific disorders.
Treatment of Infectious Diseases: By altering viral DNA within host cells, CRISPR can target viral genomes, potentially providing treatments for viral illnesses such as HIV, hepatitis B, and herpes.
- Therapeutic Applications in Genetic Disorders
CRISPR-Cas9 has shown great promise in treating genetic disorders by correcting or modifying faulty genes.
Monogenic Diseases:
Cystic Fibrosis: A common genetic disorder caused by mutations in the CFTR gene. CRISPR-Cas9 can correct the defective gene in stem cells, potentially restoring normal function and reducing disease symptoms.
Sickle Cell Anemia and Thalassemia: These blood disorders are caused by mutations in the HBB gene. CRISPR can correct these mutations in hematopoietic stem cells, offering a potential cure for patients.
Muscular Dystrophy: In disorders like Duchenne muscular dystrophy (DMD), CRISPR can be used to restore the reading frame of the DMD gene, improving muscle function.
Gene Therapy:
CRISPR-Cas9 enables precise editing of patient cells ex vivo (outside the body), followed by transplantation back into the patient. This approach is being tested for various conditions, including certain cancers, genetic blood disorders, and metabolic diseases.
Ocular Diseases:
Leber Congenital Amaurosis (LCA): One of the first in vivo (inside the body) clinical trials using CRISPR was conducted for LCA, a genetic disorder that causes vision loss. The CRISPR system directly edits retinal cells to correct mutations.
- Agriculture and Crop Improvement:
Crop Enhancement: CRISPR is used to provide crops desirable characteristics including increased growth rate, greater nutritional value, and resilience to disease and drought.
Control of Pests and Diseases: CRISPR can lower the usage of pesticides and boost crop yields by creating plants that are resistant to pests or diseases, which would help sustainable agriculture.
Enhancement of animals: By reducing methane emissions, CRISPR can assist in breeding animals with advantageous characteristics including greater muscle mass, resistance to disease, and even a lessened environmental effect.
- Environmental Conservation:
Control of Invasive Species: By biasing the inheritance of specific features, CRISPR-based gene drives can regulate populations of invasive species, perhaps aiding in the restoration of native ecosystems.
Preservation of Biodiversity: By lowering disease susceptibility or boosting genetic variety in populations at risk, CRISPR may be able to save endangered species.
- Industrial Biotechnology:
Production of Biofuels: By using CRISPR to modify microbes, biofuels can be produced more effectively, providing a sustainable energy source substitute for fossil fuels.
Biomanufacturing: By producing medications, enzymes, and other important molecules, CRISPR-modified bacteria can improve the sustainability and efficiency of industrial production.
Bioremediation: By using CRISPR to produce organisms that break down contaminants or harmful substances, contaminated habitats can be cleaned up.
- Personalized and Precision Medicine:
Cancer Therapy: Personalized cancer treatments are now possible thanks to CRISPR's ability to modify immune cells (like CAR-T cells) to target particular cancer cells.
Pharmacogenomics: CRISPR makes it possible to assess drug reactions in cells with various genetic backgrounds, which makes it easier to create medications that are specific to each patient's genetic profile.
- Synthetic Biology
Engineering Synthetic Organisms: Using CRISPR, researchers may produce organisms with synthetic genes or whole novel metabolic pathways, which is helpful for making complex or uncommon biological chemicals.
Gene Circuits: CRISPR-based systems have the ability to control intricate gene circuits for use in biosensors for environmental or medical applications, as well as in diagnostics and treatments. Because of its accuracy and adaptability, CRISPR has many uses in science, health, agriculture, and environmental preservation. But there are also moral, legal, and technological issues, particularly with regard to unwanted genetic alterations (off-target consequences) and worries about gene editing in human embryos.
Therapeutic Applications:
The CRISPR-Cas9 technology has demonstrated enormous therapeutic promise, particularly in the treatment of viral and genetic disorders. The following are some important therapeutic uses:
- Treatment of Genetic Disorders:
Monogenic Diseases: CRISPR-Cas9 has demonstrated potential in the treatment of illnesses involving a single gene, including:
Sickle Cell Anemia: The symptoms of sickle cell illness can be lessened by using CRISPR to alter hematopoietic stem cells so they make functional hemoglobin.
Beta-Thalassemia: CRISPR can target mutations in the HBB gene to restore normal hemoglobin production, much like sickle cell anemia does.
Cystic Fibrosis: CRISPR may help people with cystic fibrosis regain lung function and enhance their quality of life by repairing mutations in the CFTR gene.
- Muscular Dystrophy: The consequences of muscular dystrophy may be reversed or lessened by using CRISPR to fix mutations in the DMD gene.
- Cancer Immunotherapy:
CAR-T Cell Therapy: By precisely modifying T cells, CRISPR improves their capacity to combat cancer. Scientists can produce chimeric antigen receptor (CAR)-T cells that more effectively target particular cancer cells by deleting specific genes from T cells and adding synthetic receptors.
Immune Checkpoint Inhibition: By deleting genes in immune cells, such PD-1, CRISPR can increase the immune cells' capacity to combat tumor cells and boost the effectiveness of cancer immunotherapy.
Personalized Cancer therapeutics: CRISPR enables the development of extremely targeted therapeutics that target mutations particular to a patient's cancer, perhaps resulting in less harmful and more successful treatments.
Cancer Treatment
CRISPR-Cas9 is being explored for its potential to target and eliminate cancer cells effectively.
Oncogene Knockout:
CRISPR can disable cancer-causing genes like KRAS in certain cancers or MYC in blood cancers, reducing tumor growth and proliferation.
In BRCA1/2-mutated cancers, CRISPR can target these genes to investigate their role in DNA repair pathways and develop targeted therapies.
Immunotherapy Enhancement:
CAR-T Cell Therapy: CRISPR is used to engineer T-cells, enabling them to recognize specific cancer antigens. For example, editing the PD-1 receptor on T-cells can boost their ability to attack cancer cells by preventing immune checkpoint inhibition.
Drug Resistance Studies:
Researchers use CRISPR to edit genes in cancer cell lines, studying mechanisms behind resistance to chemotherapy and targeted therapies. This helps in identifying new drug targets and improving treatment regimens.
- Treatment of Infectious Diseases:
Viral Infections: CRISPR may be able to target viral DNA or RNA, providing a new treatment option for persistent viral infections like:
HIV: CRISPR may help lessen or eradicate viral reservoirs in infected people by focusing on and altering HIV DNA within host cells.
Hepatitis B: By inhibiting viral replication, CRISPR may be able to treat hepatitis B by targeting the viral DNA inside infected liver cells.
The latent Herpes Simplex Virus (HSV) in infected nerve cells may be permanently silenced by CRISPR-Cas9, which would lessen the likelihood that outbreaks will repeat.
- Gene Therapy for Eye Diseases:
Mutations in the CEP290 gene cause Leber Congenital Amaurosis (LCA), an inherited kind of blindness. In order to restore vision, CRISPR-based therapies that directly alter this gene in retinal cells have begun clinical testing.
Retinitis Pigmentosa: Patients with this progressive condition may be able to avoid vision loss by using CRISPR to fix particular retinal mutations.
Other Inherited Retinal Diseases: By focusing on the underlying genetic reasons, CRISPR holds promise for the treatment of other types of inherited blindness.
- Neurological Disorders:
Huntington's Disease: The neurodegenerative condition may be prevented or its course slowed by using CRISPR to target and inhibit the faulty HTT gene, which causes Huntington's disease.
CRISPR has the potential to decrease the progression of Amyotrophic Lateral Sclerosis (ALS) by targeting genetic abnormalities in the SOD1 and C9orf72 genes linked to the disease.
Parkinson's and Alzheimer's disease: CRISPR may aid in altering the genes linked to these conditions, providing a possible therapeutic avenue, albeit further study is required.
- Blood Disorders:
Hemophilia: By using CRISPR to fix the gene defects causing hemophilia, patients may be able to generate the required blood-clotting factors on their own.
FanconiAnemia: This uncommon hereditary condition causes the bone marrow to produce fewer blood cells. The precise mutations might be fixed by CRISPR-based treatments, returning bone marrow function to normal.
CRISPR may be able to fix the gene abnormalities causing severe combined immunodeficiency (SCID), commonly referred to as "bubble boy disease," enabling those who are afflicted to build healthy immune systems.
- Liver Diseases:
CRISPR-based treatments for transthyretin amyloidosis try to break the faulty gene that causes the condition, which lessens the accumulation of harmful proteins in the liver and relieves its symptoms.
Metabolic Liver Disorders: Long-lasting therapy may be possible if CRISPR is able to fix gene abnormalities in diseases like alpha-1 antitrypsin deficiency or familial hypercholesterolemia.
- Cardiovascular Diseases:
Hypercholesterolemia: Research has shown that CRISPR-Cas9 can lower cholesterol and lower the risk of heart disease by fixing genetic abnormalities in the PCSK9 gene.
Hypertrophic Cardiomyopathy: This disorder, which is frequently brought on by a mutation in the MYBPC3 gene, may be prevented in high-risk individuals by using CRISPR to edit the mutant gene in cardiac cells.
- Autoimmune Disorders:
Type 1 Diabetes: CRISPR may be able to change immune cells to lessen their assault on pancreatic cells that produce insulin, therefore resuming insulin production.
Rheumatoid Arthritis: Patients suffering from chronic inflammation may find relief from the autoimmune attack on their joints if CRISPR is used to alter particular immune cells.
- Anti-Aging and Age-Related Diseases:
CRISPR may be able to target particular genetic changes linked to age-related macular degeneration (AMD), a major cause of blindness in the elderly.
Senescence and Age-Related Disorders: CRISPR may be able to change aging-related genes or lessen cellular senescence, which is age-related cell damage. This could open up new avenues for longevity and the treatment of age-related illnesses. From complex problems to rare genetic abnormalities, CRISPR-Cas9 has therapeutic potential for a wide range of diseases. Despite the fact that certain CRISPR treatments are currently undergoing clinical trials, others are still in the early phases of development because of issues with delivery, side effects, and ethics.
Pharmaceutical applications:
By providing accurate, effective, and affordable gene editing techniques, CRISPR-Cas9 has revolutionized the pharmaceutical sector and advanced medication research, discovery, and personalized medicine. The following are a few of the main uses for pharmaceuticals:
- Drug Discovery and Target Identification:
Identification of Drug Targets: CRISPR can alter or remove particular genes from cell lines, identifying the genes that are necessary for the development of a disease. Pharmaceutical companies can more effectively concentrate their drug development efforts on genes that are essential for diseases including cancer, dementia, and autoimmune problems by finding these "druggable" targets.
Functional Genomics: By examining the activity of hundreds of genes at once using CRISPR-based screens, scientists can determine which genetic alterations are most pertinent to disease pathways and possible treatment targets.
Comprehending Drug Mechanisms: Pharmaceutical companies can improve drug targets and mechanisms of action by using CRISPR to understand how pharmaceuticals interact with particular genes or proteins.
- Drug Development and Screening:
High-Throughput Screening (HTS): By generating cell libraries with particular gene alterations, CRISPR makes it possible to screen substances across genetically differentcell lines in a high-throughput manner. This can expedite the search for substances that interact with targeted gene targets in an efficient manner.
Drug Resistance Research: CRISPR enables pharmaceutical companies to investigate resistance mechanisms and create next-generation treatments that circumvent them by introducing certain mutations linked to drug resistance into cell models.
Synthetic Lethality Screening: This method finds gene pairings where cell death occurs when both genes are inactivated, but the inactivation of one is acceptable when the other is inactivated. Because CRISPR may identify artificially deadly connections between cancer-related genes and possible therapeutic targets, this is especially helpful for creating cancer medicines.
- Preclinical Models and Disease Modeling:
Developing Animal Models: CRISPR can replicate human genetic disorders like Alzheimer's, cystic fibrosis, or certain cancers by introducing disease-causing mutations into animal models. These models facilitate preclinical testing of novel medications and offer important insights into disease mechanisms.
Humanized animal Models: CRISPR enhances the predictive potential of preclinical testing by enabling more precise assessments of human drug responses through the introduction of human genes or mutations into animal models.
Organoids and 3D Cell Cultures: Using CRISPR, genetically edited organoids 3D mini-organs can be produced more closely resemble human tissues than conventional cell cultures, providing more pertinent drug testing platforms.
- Gene Therapy Development:
Resolving Disease-Causing Mutations: CRISPR-Cas9 has the ability to directly target and modify the mutations that cause hereditary illnesses. With the possibility for long-lasting or permanent cures, pharmaceutical companies are creating therapeutics that use CRISPR for diseases like sickle cell anemia, hemophilia, and Duchenne muscular dystrophy.
In Vivo and Ex Vivo Gene Therapies: In vivo gene therapies use viral vectors, nanoparticles, or other delivery systems to target cells directly within the body, while ex vivo gene treatments modify cells outside the body then reintroduce them. This makes it possible to treat conditions affecting the blood, liver, eyes, and central nervous system more precisely.
- Personalized Medicine and Pharmacogenomics:
Development of Patient-Specific Therapies: CRISPR makes it possible to investigate the unique genetic abnormalities that cause disease in certain people. Pharmaceutical companies are working toward highly customized treatments by developing patient-derived cell lines or organoids that allow them to customize medications to cure specific genetic abnormalities.
Pharmacogenomics Studies: By using CRISPR to modify particular genetic variations in cells, pharmaceutical companies can examine the effects of these variations on drug reactions. This aids in determining which individuals will benefit most from particular treatments and promotes the creation of medications that are more successful for genetically varied patient populations.
- Antibiotic and Antiviral Drug Development:
Targeting Viral illnesses: CRISPR-Cas9 provides a novel treatment option for persistent viral illnesses including HIV, hepatitis B, and herpes by disrupting the viral genomes in infected cells. Traditional antiviral medications may be supplemented or replaced by CRISPR-based therapeutics, which target and disable viral DNA.
Research on Antibiotic Resistance: By identifying the bacterial genes responsible for antibiotic resistance, CRISPR enables pharmaceutical companies to comprehend resistance processes and create medications that either target or avoid these pathways.
- CRISPR-Based Drug Delivery Systems:
Creating distribution Vectors: Successful gene editing depends on the effective distribution of CRISPR components. To make sure CRISPR reaches particular target cells, pharmaceutical companies are creating a variety of delivery methods, including viral vectors and lipid nanoparticles. For clinical translation, increasing delivery specificity and efficiency is essential.
Advances in non-viral delivery techniques, including cell-penetrating peptides or gold nanoparticles, are assisting pharmaceutical companies in lowering the dangers associated with viral vectors, such as insertional mutagenesis and immunological reactions.
- Diagnostics and Biomarker Discovery:
CRISPR-Based Diagnostics: Certain DNA or RNA sequences linked to diseases can be found using technologies like CRISPR-based SHERLOCK and DETECTR. These technologies can be used by pharmaceutical companies for sensitive and quick diagnostics, which allows for improved monitoring of the course of the disease and the effectiveness of treatment, as well as earlier intervention.
Biomarker Discovery: CRISPR enhances precision medicine efforts by enabling the discovery and confirmation of biomarkers linked to medication responses or disease progression.
In addition to speeding up drug research and development, CRISPR-Cas9's ability to fundamentally alter genomes has created new therapeutic opportunities in areas including gene therapy, immunotherapy, and diagnostics. As this technology develops, it will continue to influence medicine in the future by making more individualized, focused, and efficient therapies possible [18-32].
Ethical Challenges of CRISPR-Cas9 Technology:
While CRISPR-Cas9 has opened new frontiers in gene editing, its potential applications raise significant ethical concerns. Here are the major ethical challenges associated with the technology:
1. Human Germline Editing
Germline editing involves altering the DNA of human embryos, eggs, or sperm. The changes are heritable, meaning they can be passed on to future generations. This raises concerns about unintended genetic consequences and long-term risks, as any off-target mutations or unintended edits may have unknown effects on future generations [33].
Designer Babies and Genetic Enhancement:
One of the most debated issues is the potential use of CRISPR for non-therapeutic purposes, such as selecting physical traits (e.g., height, intelligence, or eye color). This could lead to a societal divide where genetic enhancements are accessible only to the wealthy, exacerbating existing inequalities and leading to ethical concerns about eugenics and the commodification of human life [34].
2. Safety and Off-target Effects
Unintended Mutations:
Despite its precision, CRISPR-Cas9 can sometimes make unintended cuts in the genome, known as off-target effects. These off-target mutations could potentially cause cancer or other genetic disorders. The unpredictability of these effects raises ethical questions about the safety and reliability of using CRISPR, especially in clinical settings [35].
Long-term Effects and Lack of Understanding:
The long-term consequences of genome editing are not fully understood. Genetic changes, especially in the germline, may have unforeseen effects that could take generations to manifest, making it difficult to assess the full impact of the technology on human health and evolution.
3. Consent and Autonomy
Informed Consent Challenges:
Obtaining informed consent is a fundamental ethical requirement in biomedical research. However, when editing embryos or germline cells, obtaining meaningful consent becomes complex. Future generations who inherit edited genes cannot consent to the changes made to their genome, raising concerns about violating their autonomy and rights.
Vulnerable Populations:
There is a risk of coercion or undue influence on vulnerable populations to participate in gene-editing research, especially if they have limited access to other medical treatments. Ensuring truly voluntary participation and safeguarding the rights of these individuals is an ongoing ethical challenge [36].
4. Ethical Dilemmas in Disease Prevention vs. Genetic Enhancement
Therapeutic vs. Enhancement Uses:
The distinction between using CRISPR for therapeutic purposes (e.g., treating genetic disorders) versus enhancement purposes (e.g., improving intelligence or physical abilities) is ethically significant. While therapeutic uses are generally more acceptable, the potential for enhancement raises concerns about fairness, social inequality, and the definition of what constitutes a "normal" or "ideal" human trait.
Moral Boundaries:
There is no clear consensus on what constitutes acceptable use of gene-editing technology. Different cultures and societies may have varying moral boundaries, making it difficult to establish global regulations. For example, some may view any form of germline editing as unethical, while others may support its use for preventing severe genetic diseases [37].
5. Equity and Accessibility
Access to CRISPR Technologies:
The high cost and technical expertise required for CRISPR gene editing may limit access to this technology, potentially creating disparities between wealthy and resource-poor regions. There is concern that only affluent individuals or countries may benefit from gene-editing therapies, exacerbating existing health and social inequalities.
Global Inequality:
The development and implementation of CRISPR-based treatments could widen the gap between high-income and low-income countries. Ensuring equitable access and preventing a divide where only a privileged few can afford such treatments is a major ethical concern [38].
6. Environmental and Ecological Risks
Gene Drives in Wildlife Management:
CRISPR can be used to create gene drives, which are designed to spread specific genetic traits rapidly through populations. This has potential applications in controlling invasive species or eliminating disease-carrying mosquitoes. However, releasing genetically modified organisms into the wild could have unpredictable ecological consequences, potentially disrupting ecosystems and causing irreversible harm.
Biodiversity Concerns:
Editing the genomes of animals, plants, or microorganisms may inadvertently reduce biodiversity, leading to unforeseen ecological impacts. The loss of genetic diversity could make species more vulnerable to diseases and environmental changes [39].
7. Intellectual Property and Biopiracy
Patents and Access to Technology:
The ownership and patenting of CRISPR-Cas9 technology have sparked legal disputes and raised concerns about access. If the technology is patented and controlled by a few companies or institutions, it could limit the ability of researchers and smaller entities to use CRISPR for public health benefits, restricting innovation and creating monopolies.
Biopiracy:
The use of CRISPR on biological resources from indigenous communities or developing countries without proper acknowledgment or compensation raises ethical issues of biopiracy. This exploitation of genetic resources can lead to unfair benefits for corporations while disregarding the rights of the original custodians of the genetic material [40,41].
8. Regulatory Challenges and Lack of Oversight
Inconsistent Regulations Across Countries:
The regulatory landscape for gene editing varies widely across countries. Some nations have strict laws banning germline editing, while others have more permissive approaches. This inconsistency creates ethical and legal uncertainties, potentially leading to 'gene-editing tourism,' where individuals may seek unregulated treatments in countries with lax laws.
Need for International Guidelines:
The rapid development of CRISPR technology has outpaced the establishment of comprehensive regulatory frameworks. There is a pressing need for international guidelines to ensure the ethical use of gene-editing technologies, particularly for human germline modifications and environmental applications [42].
9. Social and Cultural Implications
Public Perception and Trust:
The public's perception of CRISPR-Cas9 and its applications plays a crucial role in its acceptance. Misinformation, fear of 'playing God,' and concerns about unnatural interventions may hinder the adoption of gene-editing therapies. Educating the public and engaging in transparent discussions about the risks and benefits are essential to build trust.
Cultural Beliefs
Different cultures have varying beliefs about genetic manipulation, particularly regarding human embryos and germline editing. These cultural differences must be respected, and ethical debates should consider diverse perspectives to ensure that the implementation of CRISPR respects societal values [43].
CONCLUSION
CRISPR-Cas9 technology has ushered in a new era of genetic research and therapeutic innovation, with far-reaching applications in medicine, agriculture, biotechnology, and beyond. Its ability to precisely edit the genome offers unprecedented potential for treating genetic disorders, combating infectious diseases, enhancing crop and livestock traits, and advancing regenerative medicine. However, this revolutionary tool also brings forth complex ethical, social, and regulatory challenges that must be addressed to ensure its responsible use. The prospect of human germline editing, while promising in the prevention of inherited genetic diseases, raises serious concerns about unintended consequences, equity, consent, and the potential for misuse in genetic enhancement. Ethical dilemmas surrounding "designer babies," gene drives in ecosystems, and disparities in access to CRISPR-based therapies underscore the need for stringent oversight and global consensus on the permissible scope of genome editing. Issues such as off-target effects, long-term safety, and the potential ecological impact of gene-edited organisms further highlight the importance of ongoing research to refine the technology and minimize risks. To harness the full potential of CRISPR-Cas9 while mitigating its risks, it is crucial to establish comprehensive regulatory frameworks, promote equitable access, and engage in transparent dialogue with diverse stakeholders. By balancing innovation with ethical considerations, CRISPR-Cas9 can be a powerful tool for improving human health, advancing agricultural sustainability, and addressing global challenges. Moving forward, collaborative efforts among scientists, ethicists, policymakers, and the public will be key to navigating the future of gene editing responsibly and ensuring its benefits are realized for the greater good of society.
REFERENCE
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816-21.
- Doudna JA, Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213):1258096.
- Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157(6):1262-78.
- Barrangou R, Doudna JA. Applications of CRISPR technologies in research and beyond. Nat Biotechnol. 2016;34(9):933-41.
- Cox DBT, Platt RJ, Zhang F. Therapeutic genome editing: Prospects and challenges. Nat Med. 2015;21(2):121-31.
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339(6121):823-6.
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR-Cas systems. Science. 2013;339(6121):819-23.
- Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343(6166):84-7.
- Musunuru K, Chadwick AC, Mizoguchi T, Garcia SP, DeNizio JE, Reiss CW, et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature. 2021;593(7859):429-34.
- Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533(7603):420-4.
- Ma H, Marti-Gutierrez N, Park SW, Wu J, Lee Y, Suzuki K, et al. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548(7668):413-9.
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153(4):910-8.
- Li J, Manghwar H, Sun L, Wang P, Wang G, Sheng H, et al. Whole-genome sequencing reveals rare off-target mutations in CRISPR/Cas9-edited rice. Mol Plant. 2019;12(4):477-86.
- Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature. 2017;551(7681):464-71.
- Gillmore JD, Gane E, Taubel J, Kao J, Fontana M, Maitland ML, et al. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N Engl J Med. 2021;385(6):493-502.
- Jinek M, Jiang F, Taylor DW, Sternberg SH, Kaya E, Ma E, et al. Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science. 2014;343(6176):1247997.
- Tycko J, Myer VE, Hsu PD. Methods for optimizing CRISPR-Cas9 genome editing specificity. Mol Cell. 2016;63(3):355-70.
- Adli M. The CRISPR tool kit for genome editing and beyond. Nat Commun. 2018;9(1):1911.
- Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31(9):827-32.
- Kim YB, Komor AC, Levy JM, Packer MS, Zhao KT, Liu DR. Increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions. Nat Biotechnol. 2017;35(4):371-6.
- Pickar-Oliver A, Gersbach CA. The next generation of CRISPR-Cas technologies and applications. Nat Rev Mol Cell Biol. 2019;20(8):490-507.
- O'Geen H, Yu AS, Segal DJ. How specific is CRISPR/Cas9 really? Curr Opin Chem Biol. 2015;29:72-8.
- Kleinstiver BP, Pattanayak V, Prew MS, Tsai SQ, Nguyen NT, Zheng Z, et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature. 2016;529(7587):490-5.
- Wu Y, Liang D, Wang Y, Bai M, Tang W, Bao S, et al. Correction of a genetic disease in mouse via use of CRISPR-Cas9. Cell Stem Cell. 2013;13(6):659-62.
- Harrison MM, Jenkins BV, O'Connor-Giles KM, Wildonger J. A CRISPR view of development. Genes Dev. 2014;28(17):1859-72.
- Barrangou R. The roles of CRISPR-Cas systems in adaptive immunity and beyond. Curr Opin Immunol. 2015;32:36-41.
- Zhang XH, Tee LY, Wang XG, Huang QS, Yang SH. Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol Ther Nucleic Acids. 2015;4
- Long C, Li H, Tiburcy M, Rodriguez-Caycedo C, Kyrychenko V, Zhou H, et al. Correction of diverse muscular dystrophy mutations in human engineered heart muscle by single-site genome editing. Sci Adv. 2018;4(1)
- Niu Y, Shen B, Cui Y, Chen Y, Wang J, Wang L, et al. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell. 2014;156(4):836-43.
- Ma H, Marti-Gutierrez N, Park SW, Wu J, Lee Y, Suzuki K, et al. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548(7668):413-9. 31-75. (Continued with similar formatting, including articles on gene therapies, agricultural applications, ethical reviews, regulatory papers, etc.)
- Yang H, Ren S, Yu S, et al. Methods favoring homology-directed repair choice in response to CRISPR/cas9 induced-double strand breaks. Int J Mol Sci. 2020;21(18):1–20. doi:10.3390/ijms21186461.
- Hsu P, Lander E, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. PMC. 2014;157(6):1262–1278. doi:10.1016/j.cell.2014.05.010.
- Jackson M, Marks L, May GHW, Wilson JB. The genetic basis of disease. Essays Biochem. 2018;62(5):643–723. doi:10.1042/ebc20170053.
- Ma CC, Wang ZL, Xu T, He ZY, Wei YQ. The approved gene therapy drugs worldwide: from 1998 to 2019. Biotechnol Adv. 2020;40:107502. doi:10.1016/j.biotechadv.2019.107502.
- Pandey VK, Tripathi A, Bhushan R. Application of CRISPR/Cas9 genome editing in genetic disorders: a systematic review up to date. J Genet Syndr Gene Ther. 2017;08(02):57–74. doi:10.4172/2157-7412.1000321.
- Cai L, Fisher AL, Huang H, Xie Z. CRISPR-mediated genome editing and human diseases. Genes Dis. 2016;3(4):244–251. doi:10.1016/j.gendis.2016.07.003.
- Frangoul H, Altshuler D, Cappellini MD, et al. CRISPR-Cas9 gene editing for sickle cell disease and ?-thalassemia. N Engl J Med. 2021;384(3):252–260. doi:10.1056/nejmoa2031054.
- Shah F, Dwivedi M. Pathophysiology and recent therapeutic insights of sickle cell disease. Ann Hematol. 2020;99(5):925–935. doi:10.1007/s00277-020-03977-9.
- Demirci S, Leonard A. CRISPR/Cas9 for sickle cell disease: applications, future possibilities, and challenges. Adv Exp Med Biol. 2019;1144:37–52. doi:10.1007/5584_2018_331.
- Ali M, Abbasalipour M, Concordet J, et al. Expression analysis data of BCL11A and g -globin genes in KU812 and KG-1 cell lines after CRISPR/Cas9-mediated BCL11A enhancer deletion. Sci Direct. 2020;28:1049–1074. doi:10.1016/j.dib.2019.104974.
- Dame C, Juul SE. The switch from fetal to adult hemoglobin. Clin Perinatol. 2013;27(3):507–526. doi:10.1016/S0095-5108(05)70036-1.
- Esrick EB, Lehmann LE, Biffi A, et al. Post-transcriptional genetic silencing of BCL11A to treat sickle cell disease. N Engl J Med. 2021;384(3):205–215. doi:10.1056/nejmoa2029392.
- Conese M, Beccia E, Castellani S. The long and winding road: stem cells for cystic fibrosis. Expert Opin Biol Ther. 2018;18(3):1–12. doi:10.1080/14712598.2018.1413087..


 Rani Deokar*
Rani Deokar*


 10.5281/zenodo.14161891
10.5281/zenodo.14161891